Atrial Fibrillation Screen, Management And Guideline Recommended Therapy (AF SMART II) in the rural primary care setting: an implementation study protocol
- PMID: 30385444
- PMCID: PMC6252758
- DOI: 10.1136/bmjopen-2018-023130
Atrial Fibrillation Screen, Management And Guideline Recommended Therapy (AF SMART II) in the rural primary care setting: an implementation study protocol
Abstract
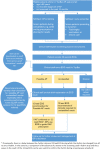
Introduction: Screening for atrial fibrillation (AF) in people ≥65 years is now recommended by guidelines and expert consensus. While AF is often asymptomatic, it is the most common heart arrhythmia and is associated with increased risk of stroke. Early identification and treatment with oral anticoagulants can substantially reduce stroke risk. The general practice setting is ideal for opportunistic screening and provides a natural pathway for treatment for those identified.This study aims to investigate the feasibility of implementing screening for AF in rural general practice using novel electronic tools. It will assess whether screening will fit within an existing workflow to quickly and accurately identify AF, and will potentially inform a generalisable, scalable approach.
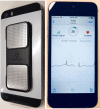
Methods and analysis: Screening with a smartphone ECG will be conducted by general practitioners and practice nurses in rural general practices in New South Wales, Australia for 3-4 months during 2018-2019. Up to 10 practices will be recruited, and we aim to screen 2000 patients aged ≥65 years. Practices will be given an electronic screening prompt and electronic decision support to guide evidence-based treatment for those with AF. De-identified data will be collected using a clinical audit tool and qualitative interviews will be conducted with selected practice staff. A process evaluation and cost-effectiveness analysis will also be undertaken. Outcomes include implementation success (proportion of eligible patients screened, fidelity to protocol), proportion of people screened identified with new AF and rates of treatment with anticoagulants and antiplatelets at baseline and completion. Results will be compared against an earlier metropolitan study and a 'control' dataset of practices.
Ethics and dissemination: Ethics approval was received from the University of Sydney Human Research Ethics Committee on 27 February 2018 (Project no.: 2017/1017). Results will be disseminated through various forums, including peer-reviewed publication and conference presentations.
Trial registration number: ACTRN12618000004268; Pre-results.
Keywords: atrial fibrillation; electronic decision support; oran anticoagulants; screening.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NL and BF report grants from BMS/Pfizer during the conduct of the study. RW and AP report other financial interests from George Health, outside the submitted work. CF reports personal fees from Pfizer outside the submitted work. LN reports grants from Pfizer/BMS and Boehringer Ingelheim outside the submitted work. BF reports prior fees and advisory board honoraria from Bayer Pharma AG, Boehringer Ingelheim, and BMS/Pfizer but for the past year has removed himself from pharmaceutical advisory boards and receives speaker fees only for accredited educational meetings.
Figures
Similar articles
-
Atrial Fibrillation Screen, Management, and Guideline-Recommended Therapy in the Rural Primary Care Setting: A Cross-Sectional Study and Cost-Effectiveness Analysis of eHealth Tools to Support All Stages of Screening.J Am Heart Assoc. 2020 Sep 15;9(18):e017080. doi: 10.1161/JAHA.120.017080. Epub 2020 Aug 31. J Am Heart Assoc. 2020. PMID: 32865129 Free PMC article.
-
Uptake of a primary care atrial fibrillation screening program (AF-SMART): a realist evaluation of implementation in metropolitan and rural general practice.BMC Fam Pract. 2019 Dec 6;20(1):170. doi: 10.1186/s12875-019-1058-9. BMC Fam Pract. 2019. PMID: 31810441 Free PMC article.
-
eHealth Tools to Provide Structured Assistance for Atrial Fibrillation Screening, Management, and Guideline-Recommended Therapy in Metropolitan General Practice: The AF - SMART Study.J Am Heart Assoc. 2019 Jan 8;8(1):e010959. doi: 10.1161/JAHA.118.010959. J Am Heart Assoc. 2019. PMID: 30590964 Free PMC article. Clinical Trial.
-
Screening for undiagnosed atrial fibrillation.Expert Rev Cardiovasc Ther. 2018 Aug;16(8):591-598. doi: 10.1080/14779072.2018.1496018. Epub 2018 Jul 16. Expert Rev Cardiovasc Ther. 2018. PMID: 29963930 Review.
-
Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration.Circulation. 2017 May 9;135(19):1851-1867. doi: 10.1161/CIRCULATIONAHA.116.026693. Circulation. 2017. PMID: 28483832 Review.
Cited by
-
2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society.J Arrhythm. 2021 Jan 29;37(2):271-319. doi: 10.1002/joa3.12461. eCollection 2021 Apr. J Arrhythm. 2021. PMID: 33850572 Free PMC article.
-
Atrial fibrillation (AF) pilot screening programme in primary care in Ireland: an implementation study protocol.BMJ Open. 2022 Feb 7;12(2):e054324. doi: 10.1136/bmjopen-2021-054324. BMJ Open. 2022. PMID: 35131828 Free PMC article.
-
2021 ISHNE / HRS / EHRA / APHRS Collaborative Statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology / Heart Rhythm Society / European Heart Rhythm Association / Asia Pacific Heart Rhythm Society.Eur Heart J Digit Health. 2021 Jan 29;2(1):7-48. doi: 10.1093/ehjdh/ztab001. eCollection 2021 Mar. Eur Heart J Digit Health. 2021. PMID: 36711170 Free PMC article.
-
2021 ISHNE/ HRS/ EHRA/ APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society.Ann Noninvasive Electrocardiol. 2021 Mar;26(2):e12795. doi: 10.1111/anec.12795. Epub 2021 Jan 29. Ann Noninvasive Electrocardiol. 2021. PMID: 33513268 Free PMC article.
-
Considering User Experience and Behavioral Approaches in the Design of mHealth Interventions for Atrial Fibrillation: Systematic Review.J Med Internet Res. 2024 Oct 4;26:e54405. doi: 10.2196/54405. J Med Internet Res. 2024. PMID: 39365991 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
