Vorinostat, a pan-HDAC inhibitor, abrogates productive HPV-18 DNA amplification
- PMID: 30385631
- PMCID: PMC6255162
- DOI: 10.1073/pnas.1801156115
Vorinostat, a pan-HDAC inhibitor, abrogates productive HPV-18 DNA amplification
Abstract
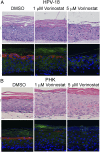
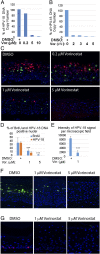
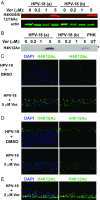
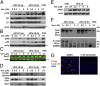
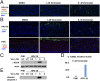
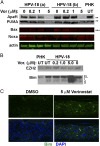
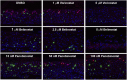
Human papillomaviruses (HPVs) cause epithelial proliferative diseases. Persistent infection of the mucosal epithelia by the high-risk genotypes can progress to high-grade dysplasia and cancers. Viral transcription and protein activities are intimately linked to regulation by histone acetyltransferases and histone deacetylases (HDACs) that remodel chromatin and regulate gene expression. HDACs are also essential to remodel and repair replicating chromatin to enable the progression of replication forks. As such, Vorinostat (suberoylanilide hydroximic acid), and other pan-HDAC inhibitors, are used to treat lymphomas. Here, we investigated the effects of Vorinostat on productive infection of the high-risk HPV-18 in organotypic cultures of primary human keratinocytes. HPV DNA amplifies in the postmitotic, differentiated cells of squamous epithelia, in which the viral oncoproteins E7 and E6 establish a permissive milieu by destabilizing major tumor suppressors, the pRB family proteins and p53, respectively. We showed that Vorinostat significantly reduced these E6 and E7 activities, abrogated viral DNA amplification, and inhibited host DNA replication. The E7-induced DNA damage response, which is critical for both events, was also compromised. Consequently, Vorinostat exposure led to DNA damage and triggered apoptosis in HPV-infected, differentiated cells, whereas uninfected tissues were spared. Apoptosis was attributed to highly elevated proapoptotic Bim isoforms that are known to be repressed by EZH2 in a repressor complex containing HDACs. Two other HDAC inhibitors, Belinostat and Panobinostat, also inhibited viral DNA amplification and cause apoptosis. We suggest that HDAC inhibitors are promising therapeutic agents to treat benign HPV infections, abrogate progeny virus production, and hence interrupt transmission.
Keywords: HDAC inhibitors; HPV DNA amplification; HPV E6 and E7 activities; apoptosis; organotypic cultures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Stoler MH, Wolinsky SM, Whitbeck A, Broker TR, Chow LT. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989;172:331–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

