Dose-Dependent Effect of Enzyme Replacement Therapy on Neutralizing Antidrug Antibody Titers and Clinical Outcome in Patients with Fabry Disease
- PMID: 30385651
- PMCID: PMC6287863
- DOI: 10.1681/ASN.2018070740
Dose-Dependent Effect of Enzyme Replacement Therapy on Neutralizing Antidrug Antibody Titers and Clinical Outcome in Patients with Fabry Disease
Abstract
Background: Use of enzyme replacement therapy (ERT) to treat Fabry disease, caused by deficient lysosomal α-galactosidase A activity, can lead to formation of neutralizing antidrug antibodies (ADAs). These antibodies are associated with increased accumulation of plasma globotriaosylceramide (Gb3) and disease progression. Because agalsidase ERT can saturate ADA-binding sites during infusions (achieving agalsidase/antibody equilibrium), we investigated in this open cohort study whether saturated patients (who have excess agalsidase after infusions) experience better clinical outcomes compared with not saturated patients (who have excess ADAs after infusions).
Methods: We isolated ADAs from sera of 26 men with Fabry disease receiving ERT (for a median of 94 months) and determined the amount of agalsidase necessary for antibody saturation. Clinical and biochemical outcomes included measurements of eGFR, interventricular septum thickness, and lyso-Gb3.
Results: ADA titers decreased significantly in all patients during infusion. Agalsidase-α and agalsidase-β had similar ADA-binding capacity and comparable ADA saturation frequency. Fourteen patients with saturated ADAs presented with mild (but significant) loss of eGFR, stable septum thickness, and significantly decreased lyso-Gb3 levels. The 12 not saturated patients had a more pronounced and significant loss of eGFR, increased septum thickness, and a smaller, nonsignificant reduction in lyso-Gb3, over time. In three patients, dose escalation resulted in partially elevated ADA titers, but importantly, also in reduced lyso-Gb3 levels.
Conclusions: A not saturated ADA status during infusion is associated with progressive loss of eGFR and ongoing cardiac hypertrophy. Dose escalation can result in saturation of ADAs and decreasing lyso-Gb3 levels, but may lead to increased ADA titers.
Keywords: Fabry disease; chronic kidney disease; complexes; glomerular filtration rate; immune; left ventricular hypertrophy.
Copyright © 2018 by the American Society of Nephrology.
Figures


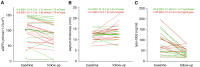


Similar articles
-
Effects of switching from agalsidase-α to agalsidase-β on biomarkers, renal and cardiac parameters, and disease severity in fabry disease forming neutralizing antidrug antibodies: a case report.CEN Case Rep. 2024 Aug;13(4):290-296. doi: 10.1007/s13730-023-00843-1. Epub 2023 Dec 22. CEN Case Rep. 2024. PMID: 38135868 Free PMC article.
-
Long-Term Dose-Dependent Agalsidase Effects on Kidney Histology in Fabry Disease.Clin J Am Soc Nephrol. 2017 Sep 7;12(9):1470-1479. doi: 10.2215/CJN.01820217. Epub 2017 Jun 16. Clin J Am Soc Nephrol. 2017. PMID: 28625968 Free PMC article.
-
Efficacy and safety of enzyme-replacement-therapy with agalsidase alfa in 36 treatment-naïve Fabry disease patients.BMC Pharmacol Toxicol. 2017 Jun 7;18(1):43. doi: 10.1186/s40360-017-0152-7. BMC Pharmacol Toxicol. 2017. PMID: 28592315 Free PMC article.
-
Current status of the immunogenicity of enzyme replacement therapy in fabry disease.Orphanet J Rare Dis. 2025 May 26;20(1):253. doi: 10.1186/s13023-025-03705-4. Orphanet J Rare Dis. 2025. PMID: 40420145 Free PMC article. Review.
-
Mechanisms of Neutralizing Anti-drug Antibody Formation and Clinical Relevance on Therapeutic Efficacy of Enzyme Replacement Therapies in Fabry Disease.Drugs. 2021 Nov;81(17):1969-1981. doi: 10.1007/s40265-021-01621-y. Epub 2021 Nov 8. Drugs. 2021. PMID: 34748189 Free PMC article.
Cited by
-
2021 TSOC Expert Consensus on the Clinical Features, Diagnosis, and Clinical Management of Cardiac Manifestations of Fabry Disease.Acta Cardiol Sin. 2021 Jul;37(4):337-354. doi: 10.6515/ACS.202107_37(4).20210601A. Acta Cardiol Sin. 2021. PMID: 34257484 Free PMC article. Review.
-
Influence of Treatment Effect Modifiers in Fabry Disease: A Systematic Literature Review.Adv Ther. 2025 Feb;42(2):579-596. doi: 10.1007/s12325-024-03062-x. Epub 2024 Dec 5. Adv Ther. 2025. PMID: 39636566 Free PMC article.
-
Immunogenicity of Protein Therapeutics: A Lymph Node Perspective.Front Immunol. 2020 May 14;11:791. doi: 10.3389/fimmu.2020.00791. eCollection 2020. Front Immunol. 2020. PMID: 32477334 Free PMC article. Review.
-
Effects of Current Therapies on Disease Progression in Fabry Disease: A Narrative Review for Better Patient Management in Clinical Practice.Adv Ther. 2025 Feb;42(2):597-635. doi: 10.1007/s12325-024-03041-2. Epub 2024 Dec 5. Adv Ther. 2025. PMID: 39636569 Free PMC article. Review.
-
Developments in the treatment of Fabry disease.J Inherit Metab Dis. 2020 Sep;43(5):908-921. doi: 10.1002/jimd.12228. Epub 2020 Mar 2. J Inherit Metab Dis. 2020. PMID: 32083331 Free PMC article. Review.
References
-
- Zarate YA, Hopkin RJ: Fabry’s disease. Lancet 372: 1427–1435, 2008 - PubMed
-
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. .: International Collaborative Fabry Disease Study Group : Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 - PubMed
-
- Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, et al. .: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 - PubMed
-
- Linthorst GE, Hollak CEM, Donker-Koopman WE, Strijland A, Aerts JMFG: Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta. Kidney Int 66: 1589–1595, 2004 - PubMed
-
- Vedder AC, Breunig F, Donker-Koopman WE, Mills K, Young E, Winchester B, et al. .: Treatment of Fabry disease with different dosing regimens of agalsidase: Effects on antibody formation and GL-3. Mol Genet Metab 94: 319–325, 2008 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

