Fas activation alters tight junction proteins in acute lung injury
- PMID: 30385692
- PMCID: PMC6339809
- DOI: 10.1136/thoraxjnl-2018-211535
Fas activation alters tight junction proteins in acute lung injury
Abstract
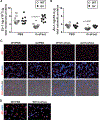
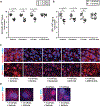
Background:The acute respiratory distress syndrome (ARDS) is characterized by protein-rich oedema in the alveolar spaces, a feature in which Fas-mediated apoptosis of the alveolar epithelium has been involved. Objective:To determine whether Fas activation increases protein permeability by mechanisms involving disruption of the paracellular tight junction (TJ) proteins in the pulmonary alveoli. Methods: Protein permeability and the expression of TJ proteins were assessed in vivo in wild-type and Fas-deficient lpr mice 16 hours after the intratracheal instillation of recombinant human soluble Fas ligand (rh-sFasL), and at different time points in vitro in human pulmonary alveolar epithelial cells (HPAEpiC) exposed to rh-sFasL Results:Activation of the Fas pathway increased protein permeability in mouse lungs and altered the expression of the TJ proteins occludin and zonula occludens-1 in the alveolar-capillary membrane in vivo and in human alveolar epithelial cell monolayers in vitro. Blockade of caspase-3, but not inhibition of tyrosine kinase dependent pathways, prevented the alterations in TJ protein expression and permeability induced by the Fas/FasL system in human alveolar cell monolayers in vitro. We also observed that both the Fas-induced increase of protein permeability and disruption of TJ proteins occurred before cell death could be detected in the cell monolayers in vitro. Conclusion:Targeting caspase pathways could prevent the disruption of TJs and reduce the formation of lung oedema in the early stages of ARDS.
Keywords: ards; innate immunity; pulmonary oedema.
© Author(s) (or their employer(s)) 2019. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



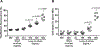


References
-
- Matthay MA, and Wiener-Kronish JP 1990. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142:1250–1257. - PubMed
-
- Ware LB, and Matthay MA 2001. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. American journal of respiratory and critical care medicine 163:1376–1383. - PubMed
-
- Schneeberger EE, and Lynch RD 1992. Structure, function, and regulation of cellular tight junctions. The American journal of physiology 262:L647–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
