Role of Alström syndrome 1 in the regulation of blood pressure and renal function
- PMID: 30385718
- PMCID: PMC6238740
- DOI: 10.1172/jci.insight.95076
Role of Alström syndrome 1 in the regulation of blood pressure and renal function
Abstract
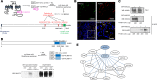
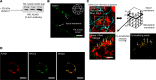
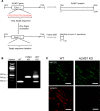
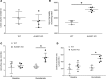
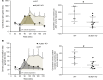
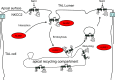
Elevated blood pressure (BP) and renal dysfunction are complex traits representing major global health problems. Single nucleotide polymorphisms identified by genome-wide association studies have identified the Alström syndrome 1 (ALMS1) gene locus to render susceptibility for renal dysfunction, hypertension, and chronic kidney disease (CKD). Mutations in the ALMS1 gene in humans causes Alström syndrome, characterized by progressive metabolic alterations including hypertension and CKD. Despite compelling genetic evidence, the underlying biological mechanism by which mutations in the ALMS1 gene lead to the above-mentioned pathophysiology is not understood. We modeled this effect in a KO rat model and showed that ALMS1 genetic deletion leads to hypertension. We demonstrate that the link between ALMS1 and hypertension involves the activation of the renal Na+/K+/2Cl- cotransporter NKCC2, mediated by regulation of its endocytosis. Our findings establish a link between the genetic susceptibility to hypertension, CKD, and the expression of ALMS1 through its role in a salt-reabsorbing tubular segment of the kidney. These data point to ALMS1 as a potentially novel gene involved in BP and renal function regulation.
Keywords: Cell Biology; Chronic kidney disease; Epithelial transport of ions and water; Protein traffic.
Conflict of interest statement
Figures



Comment in
-
ALMS1-NKCC2 interactions.Nat Rev Nephrol. 2019 Feb;15(2):62. doi: 10.1038/s41581-018-0084-5. Nat Rev Nephrol. 2019. PMID: 30443014 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

