Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema
- PMID: 30385726
- PMCID: PMC6238745
- DOI: 10.1172/jci.insight.120694
Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema
Abstract
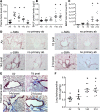
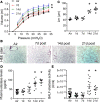
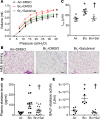
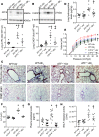
Pulmonary fibrosis and emphysema are irreversible chronic events after inhalation injury. However, the mechanism(s) involved in their development remain poorly understood. Higher levels of plasma and lung heme have been recorded in acute lung injury associated with several insults. Here, we provide the molecular basis for heme-induced chronic lung injury. We found elevated plasma heme in chronic obstructive pulmonary disease (COPD) (GOLD stage 4) patients and also in a ferret model of COPD secondary to chronic cigarette smoke inhalation. Next, we developed a rodent model of chronic lung injury, where we exposed C57BL/6 mice to the halogen gas, bromine (Br2) (400 ppm, 30 minutes), and returned them to room air resulting in combined airway fibrosis and emphysematous phenotype, as indicated by high collagen deposition in the peribronchial spaces, increased lung hydroxyproline concentrations, and alveolar septal damage. These mice also had elevated pulmonary endoplasmic reticulum (ER) stress as seen in COPD patients; the pharmacological or genetic diminution of ER stress in mice attenuated Br2-induced lung changes. Finally, treating mice with the heme-scavenging protein, hemopexin, reduced plasma heme, ER stress, airway fibrosis, and emphysema. This is the first study to our knowledge to report elevated heme in COPD patients and establishes heme scavenging as a potential therapy after inhalation injury.
Keywords: COPD; Fibrosis; Pulmonology.
Conflict of interest statement
Figures





References
-
- Grubstein A, Bendayan D, Schactman I, Cohen M, Shitrit D, Kramer MR. Concomitant upper-lobe bullous emphysema, lower-lobe interstitial fibrosis and pulmonary hypertension in heavy smokers: report of eight cases and review of the literature. Respir Med. 2005;99(8):948–954. doi: 10.1016/j.rmed.2004.12.010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

