Characterization of a recurrent missense mutation in the forkhead DNA-binding domain of FOXP1
- PMID: 30385778
- PMCID: PMC6212433
- DOI: 10.1038/s41598-018-34437-0
Characterization of a recurrent missense mutation in the forkhead DNA-binding domain of FOXP1
Erratum in
-
Author Correction: Characterization of a recurrent missense mutation in the forkhead DNA-binding domain of FOXP1.Sci Rep. 2020 Apr 15;10(1):6635. doi: 10.1038/s41598-020-62950-8. Sci Rep. 2020. PMID: 32296074 Free PMC article.
Abstract
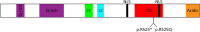
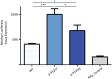
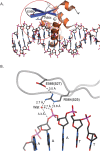
Haploinsufficiency of Forkhead box protein P1 (FOXP1), a highly conserved transcription factor, leads to developmental delay, intellectual disability, autism spectrum disorder, speech delay, and dysmorphic features. Most of the reported FOXP1 mutations occur on the C-terminus of the protein and cluster around to the forkhead domain. All reported FOXP1 pathogenic variants result in abnormal cellular localization and loss of transcriptional repression activity of the protein product. Here we present three patients with the same FOXP1 mutation, c.1574G>A (p.R525Q), that results in the characteristic loss of transcription repression activity. This mutation, however, represents the first reported FOXP1 mutation that does not result in cytoplasmic or nuclear aggregation of the protein but maintains normal nuclear localization.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
FOXP1 syndrome: a review of the literature and practice parameters for medical assessment and monitoring.J Neurodev Disord. 2021 Apr 23;13(1):18. doi: 10.1186/s11689-021-09358-1. J Neurodev Disord. 2021. PMID: 33892622 Free PMC article. Review.
-
Prospective investigation of FOXP1 syndrome.Mol Autism. 2017 Oct 24;8:57. doi: 10.1186/s13229-017-0172-6. eCollection 2017. Mol Autism. 2017. PMID: 29090079 Free PMC article.
-
Identification and functional characterization of de novo FOXP1 variants provides novel insights into the etiology of neurodevelopmental disorder.Hum Mol Genet. 2016 Feb 1;25(3):546-57. doi: 10.1093/hmg/ddv495. Epub 2015 Dec 8. Hum Mol Genet. 2016. PMID: 26647308
-
Equivalent missense variant in the FOXP2 and FOXP1 transcription factors causes distinct neurodevelopmental disorders.Hum Mutat. 2017 Nov;38(11):1542-1554. doi: 10.1002/humu.23303. Epub 2017 Aug 14. Hum Mutat. 2017. PMID: 28741757
-
Autism-like features and FOXP1 syndrome: A scoping review.Brain Dev. 2025 Jun;47(3):104346. doi: 10.1016/j.braindev.2025.104346. Epub 2025 Mar 17. Brain Dev. 2025. PMID: 40101508
Cited by
-
FOXP1 syndrome: a review of the literature and practice parameters for medical assessment and monitoring.J Neurodev Disord. 2021 Apr 23;13(1):18. doi: 10.1186/s11689-021-09358-1. J Neurodev Disord. 2021. PMID: 33892622 Free PMC article. Review.
-
Individuals with FOXP1 syndrome present with a complex neurobehavioral profile with high rates of ADHD, anxiety, repetitive behaviors, and sensory symptoms.Mol Autism. 2021 Sep 29;12(1):61. doi: 10.1186/s13229-021-00469-z. Mol Autism. 2021. PMID: 34588003 Free PMC article.
-
Adolescents and adults with FOXP1 syndrome show high rates of anxiety and externalizing behaviors but not psychiatric decompensation or skill loss.Front Psychiatry. 2025 Feb 24;16:1526383. doi: 10.3389/fpsyt.2025.1526383. eCollection 2025. Front Psychiatry. 2025. PMID: 40066139 Free PMC article.
-
EWS splicing regulation contributes to balancing Foxp1 isoforms required for neuronal differentiation.Nucleic Acids Res. 2022 Apr 8;50(6):3362-3378. doi: 10.1093/nar/gkac154. Nucleic Acids Res. 2022. PMID: 35253879 Free PMC article.
-
Case report: Expanding the phenotype of FOXP1-related intellectual disability syndrome and hyperkinetic movement disorder in differential diagnosis with epileptic seizures.Front Neurol. 2023 Jul 14;14:1207176. doi: 10.3389/fneur.2023.1207176. eCollection 2023. Front Neurol. 2023. PMID: 37521304 Free PMC article.

