Coupling the Structural and Functional Assembly of Synaptic Release Sites
- PMID: 30386217
- PMCID: PMC6198076
- DOI: 10.3389/fnana.2018.00081
Coupling the Structural and Functional Assembly of Synaptic Release Sites
Abstract
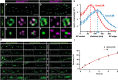
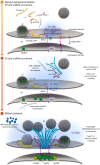
Information processing in our brains depends on the exact timing of calcium (Ca2+)-activated exocytosis of synaptic vesicles (SVs) from unique release sites embedded within the presynaptic active zones (AZs). While AZ scaffolding proteins obviously provide an efficient environment for release site function, the molecular design creating such release sites had remained unknown for a long time. Recent advances in visualizing the ultrastructure and topology of presynaptic protein architectures have started to elucidate how scaffold proteins establish "nanodomains" that connect voltage-gated Ca2+ channels (VGCCs) physically and functionally with release-ready SVs. Scaffold proteins here seem to operate as "molecular rulers or spacers," regulating SV-VGCC physical distances within tens of nanometers and, thus, influence the probability and plasticity of SV release. A number of recent studies at Drosophila and mammalian synapses show that the stable positioning of discrete clusters of obligate release factor (M)Unc13 defines the position of SV release sites, and the differential expression of (M)Unc13 isoforms at synapses can regulate SV-VGCC coupling. We here review the organization of matured AZ scaffolds concerning their intrinsic organization and role for release site formation. Moreover, we also discuss insights into the developmental sequence of AZ assembly, which often entails a tightening between VGCCs and SV release sites. The findings discussed here are retrieved from vertebrate and invertebrate preparations and include a spectrum of methods ranging from cell biology, super-resolution light and electron microscopy to biophysical and electrophysiological analysis. Our understanding of how the structural and functional organization of presynaptic AZs are coupled has matured, as these processes are crucial for the understanding of synapse maturation and plasticity, and, thus, accurate information transfer and storage at chemical synapses.
Keywords: AZ scaffold protein superfamilies; active zone assembly; calcium channel positioning; coupling distances; release sites.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

