Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases
- PMID: 30386231
- PMCID: PMC6199465
- DOI: 10.3389/fphar.2018.01048
Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases
Abstract
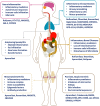
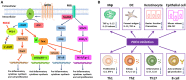
Phosphodiesterase-4 (PDE4), mainly present in immune cells, epithelial cells, and brain cells, manifests as an intracellular non-receptor enzyme that modulates inflammation and epithelial integrity. Inhibition of PDE4 is predicted to have diverse effects via the elevation of the level of cyclic adenosine monophosphate (cAMP) and the subsequent regulation of a wide array of genes and proteins. It has been identified that PDE4 is a promising therapeutic target for the treatment of diverse pulmonary, dermatological, and severe neurological diseases. Over the past decades, numerous PDE4 inhibitors have been designed and synthesized, among which roflumilast, apremilast, and crisaborole were approved for the treatment of inflammatory airway diseases, psoriatic arthritis, and atopic dermatitis, respectively. It is regrettable that the dramatic efficacies of a drug are often accompanied by adverse effects, such as nausea, emesis, and gastrointestinal reactions. However, substantial advances have been made to mitigate the adverse effects and obtain better benefit-to-risk ratio. This review highlights the dialectical role of PDE4 in drug discovery and the disquisitive details of certain PDE4 inhibitors to provide an overview of the topics that still need to be addressed in the future.
Keywords: apremilast; atopic dermatitis; crisaborole; inflammatory airway diseases; inflammatory bowel disease; phosphodiesterase-4; psoriasis; roflumilast.
Figures

References
-
- Akama T., Baker S. J., Zhang Y. K., Hernandez V., Zhou H., Sanders V., et al. . (2009). Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 19, 2129–2132. 10.1016/j.bmcl.2009.03.007 - DOI - PubMed
-
- Andoh T., Kuraishi Y. (2014). Antipruritic mechanisms of topical E6005, a phosphodiesterase 4 inhibitor: inhibition of responses to proteinase-activated receptor 2 stimulation mediated by increase in intracellular cyclic AMP. J. Dermatol. Sci. 76, 206–213. 10.1016/j.jdermsci.2014.10.005 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

