Agricultural Practices Influence Salmonella Contamination and Survival in Pre-harvest Tomato Production
- PMID: 30386314
- PMCID: PMC6198144
- DOI: 10.3389/fmicb.2018.02451
Agricultural Practices Influence Salmonella Contamination and Survival in Pre-harvest Tomato Production
Abstract
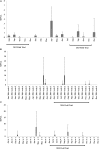
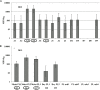
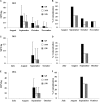
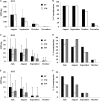
Between 2000 and 2010 the Eastern Shore of Virginia was implicated in four Salmonella outbreaks associated with tomato. Therefore, a multi-year study (2012-2015) was performed to investigate presumptive factors associated with the contamination of Salmonella within tomato fields at Virginia Tech's Eastern Shore Agricultural Research and Extension Center. Factors including irrigation water sources (pond and well), type of soil amendment: fresh poultry litter (PL), PL ash, and a conventional fertilizer (triple superphosphate - TSP), and production practices: staked with plastic mulch (SP), staked without plastic mulch (SW), and non-staked without plastic mulch (NW), were evaluated by split-plot or complete-block design. All field experiments relied on naturally occurring Salmonella contamination, except one follow up experiment (worst-case scenario) which examined the potential for contamination in tomato fruits when Salmonella was applied through drip irrigation. Samples were collected from pond and well water; PL, PL ash, and TSP; and the rhizosphere, leaves, and fruits of tomato plants. Salmonella was quantified using a most probable number method and contamination ratios were calculated for each treatment. Salmonella serovar was determined by molecular serotyping. Salmonella populations varied significantly by year; however, similar trends were evident each year. Findings showed use of untreated pond water and raw PL amendment increased the likelihood of Salmonella detection in tomato plots. Salmonella Newport and Typhimurium were the most frequently detected serovars in pond water and PL amendment samples, respectively. Interestingly, while these factors increased the likelihood of Salmonella detection in tomato plots (rhizosphere and leaves), all tomato fruits sampled (n = 4800) from these plots were Salmonella negative. Contamination of tomato fruits was extremely low (< 1%) even when tomato plots were artificially inoculated with an attenuated Salmonella Newport strain (104 CFU/mL). Furthermore, Salmonella was not detected in tomato plots irrigated using well water and amended with PL ash or TSP. Production practices also influenced the likelihood of Salmonella detection in tomato plots. Salmonella detection was higher in tomato leaf samples for NW plots, compared to SP and SW plots. This study provides evidence that attention to agricultural inputs and production practices may help reduce the likelihood of Salmonella contamination in tomato fields.
Keywords: Salmonella; agricultural practices; irrigation; poultry litter; tomato fields.
Figures


References
-
- Bell R. L., Zheng J., Burrows E., Allard S., Wang C. Y., Keys C. E., et al. (2015). Ecological prevalence, genetic diversity, and epidemiological aspects of Salmonella isolated from tomato agricultural regions of the Virginia Eastern Shore. Front. Microbiol. 6:415. 10.3389/fmicb.2015.00415 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

