New and emerging concepts in the use of denosumab for the treatment of osteoporosis
- PMID: 30386439
- PMCID: PMC6204627
- DOI: 10.1177/1759720X18805759
New and emerging concepts in the use of denosumab for the treatment of osteoporosis
Abstract
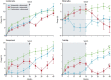
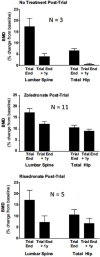
Denosumab is a fully human monoclonal antibody to receptor activator of nuclear factor kappa-B ligand (RANKL), a cytokine expressed by cells of the osteoblast lineage that is a key regulator of osteoclastic bone resorption. By binding and neutralizing RANKL, denosumab inhibits osteoclast differentiation, activity, and survival. Clinical trials in postmenopausal women with osteoporosis have shown that it reduces the risk of vertebral fractures, nonvertebral fractures, and hip fractures, with a generally favorable safety profile. With a dose of 60 mg subcutaneously every 6 months, it is approved for: treatment of postmenopausal women and men with osteoporosis, and for women and men with glucocorticoid-induced osteoporosis who are at high risk for fracture; treatment to increase bone mass in men at high risk for fracture receiving androgen-deprivation therapy for nonmetastatic prostate cancer; and treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer. Atypical femur fractures and osteonecrosis of the jaw have been reported in patients treated with denosumab. Discontinuation of denosumab is followed by rapidly rising bone turnover markers, decreasing bone density, and vertebral fracture risk that returns to baseline, with a possible increase in the risk of multiple vertebral fractures. Further study is needed to clarify this potential risk. After stopping long-term denosumab, patients should be switched to another antiresorptive agent to maintain the benefit achieved with denosumab.
Keywords: bone density; discontinuation; fracture; osteoporosis; treatment.
Conflict of interest statement
Conflict of interest statement: The author has received institutional grant/research support from Amgen, PFEnex, and Mereo; he has served on scientific advisory boards for Amgen, Radius, Shire, Alexion, Ultragenyx, and Sandoz; he serves on the speakers’ bureau for Shire, Alexion, and Radius; he is a board member of the National Osteoporosis Foundation, International Society for Clinical Densitometry, and Osteoporosis Foundation of New Mexico.
Figures
References
-
- Klibanski A, Adams-Campbell L, Bassford T, et al. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785–795. - PubMed
-
- International Osteoporosis Foundation. Facts and statistics. https://www.iofbonehealth.org/facts-statistics (accessed 29 May 2017).
-
- Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med 1997; 103: 12S–17S; discussion 17S–19S. - PubMed
-
- Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther 2015; 37: 1837–1850. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

