Design and implementation of pragmatic clinical trials using the electronic medical record and an adaptive design
- PMID: 30386852
- PMCID: PMC6207187
- DOI: 10.1093/jamiaopen/ooy017
Design and implementation of pragmatic clinical trials using the electronic medical record and an adaptive design
Abstract
Objectives: To demonstrate the feasibility of pragmatic clinical trials comparing the effectiveness of treatments using the electronic medical record (EMR) and an adaptive assignment design.
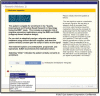
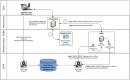
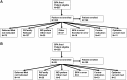
Methods: We have designed and are implementing pragmatic trials at the point-of-care using custom-designed structured clinical documentation support and clinical decision support tools within our physician's typical EMR workflow. We are applying a subgroup based adaptive design (SUBA) that enriches treatment assignments based on baseline characteristics and prior outcomes. SUBA uses information from a randomization phase (phase 1, equal randomization, 120 patients), to adaptively assign treatments to the remaining participants (at least 300 additional patients total) based on a Bayesian hierarchical model. Enrollment in phase 1 is underway in our neurology clinical practices for 2 separate trials using this method, for migraine and mild cognitive impairment (MCI).
Results: We are successfully collecting structured data, in the context of the providers' clinical workflow, necessary to conduct our trials. We are currently enrolling patients in 2 point-of-care trials of non-inferior treatments. As of March 1, 2018, we have enrolled 36% of eligible patients into our migraine study and 63% of eligible patients into our MCI study. Enrollment is ongoing and validation of outcomes has begun.
Discussion: This proof of concept article demonstrates the feasibility of conducting pragmatic trials using the EMR and an adaptive design.
Conclusion: The demonstration of successful pragmatic clinical trials based on a customized EMR and adaptive design is an important next step in achieving personalized medicine and provides a framework for future studies of comparative effectiveness.
Keywords: clinical decision support; electronic medical records; pragmatic clinical trials; precision medicine; sub-group based adaptive designs.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures
References
-
- Westrich KD, Wilhelm JA, Schur CL.. Comparative effectiveness research in the U.S.A.: When will there be an impact on healthcare decision-making? J Comp Eff Res 2016; 5 (2): 207–16. - PubMed
-
- Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials 2012; 9 (4): 436–46. - PubMed
-
- Tunis SR, Stryer DB, Clancy CM.. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003; 290 (12): 1624–32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
