The physics of radioembolization
- PMID: 30386924
- PMCID: PMC6212377
- DOI: 10.1186/s40658-018-0221-z
The physics of radioembolization
Abstract
Radioembolization is an established treatment for chemoresistant and unresectable liver cancers. Currently, treatment planning is often based on semi-empirical methods, which yield acceptable toxicity profiles and have enabled the large-scale application in a palliative setting. However, recently, five large randomized controlled trials using resin microspheres failed to demonstrate a significant improvement in either progression-free survival or overall survival in both hepatocellular carcinoma and metastatic colorectal cancer. One reason for this might be that the activity prescription methods used in these studies are suboptimal for many patients.In this review, the current dosimetric methods and their caveats are evaluated. Furthermore, the current state-of-the-art of image-guided dosimetry and advanced radiobiological modeling is reviewed from a physics' perspective. The current literature is explored for the observation of robust dose-response relationships followed by an overview of recent advancements in quantitative image reconstruction in relation to image-guided dosimetry.This review is concluded with a discussion on areas where further research is necessary in order to arrive at a personalized treatment method that provides optimal tumor control and is clinically feasible.
Keywords: Dose-effect relationship; Dosimetry; Personalized medicine; Radiobiological model; Radioembolization; Theranostics.
Conflict of interest statement
Ethics approval and consent to participate
For this type of study, formal consent is not required and informed consent is not applicable.
Consent for publication
Not applicable
Competing interests
MGEHL is a consultant for BTG International and Terumo and has received research support from Quirem Medical.The Department of Radiology and Nuclear Medicine of the UMC Utrecht receives royalties from Quirem Medical.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

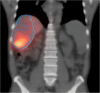


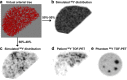
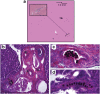
References
-
- Gibbs P, Gebski V, Van Buskirk M, Thurston K, Cade DN, Van Hazel GA. Selective internal radiation therapy (SIRT) with yttrium-90 resin microspheres plus standard systemic chemotherapy regimen of FOLFOX versus FOLFOX alone as first-line treatment of non-resectable liver metastases from colorectal cancer: the SIRFLOX study. BMC Cancer. 2014;14:897. doi: 10.1186/1471-2407-14-897. - DOI - PMC - PubMed
-
- Dutton SJ, Kenealy N, Love SB, Wasan HS, Sharma RA, FOXFIRE Protocol Development Group and the NCRI Colorectal Clinical Study Group FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional selective internal radiation therapy (SIRT) as first-line treatment for patients with unresectable liver-on. BMC Cancer. 2014;14:497. doi: 10.1186/1471-2407-14-497. - DOI - PMC - PubMed
-
- Van Hazel GA, Heinemann V, Sharma NK, Findlay MPN, Ricke J, Peeters M, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1723–1731. doi: 10.1200/JCO.2015.66.1181. - DOI - PubMed
-
- Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159–1171. doi: 10.1016/S1470-2045(17)30457-6. - DOI - PMC - PubMed
-
- Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

