Lysyl oxidase-like 2 is a regulator of angiogenesis through modulation of endothelial-to-mesenchymal transition
- PMID: 30387148
- PMCID: PMC6587725
- DOI: 10.1002/jcp.27695
Lysyl oxidase-like 2 is a regulator of angiogenesis through modulation of endothelial-to-mesenchymal transition
Abstract
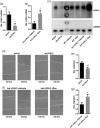
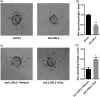
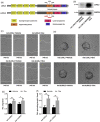
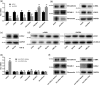
Lysyl oxidase-like 2 (LOXL2) belongs to the family of lysyl oxidases, and as such promotes crosslinking of collagens and elastin by oxidative deamination of lysine residues. In endothelial cells (ECs), LOXL2 is involved in crosslinking and scaffolding of collagen IV. Additionally, several reports have shown a role for LOXL2 in other processes, including regulation of gene expression, tumor metastasis, and epithelial-to-mesenchymal transition (EMT). Here, we demonstrate an additional role for LOXL2 in the regulation of angiogenesis by modulation of endothelial-to-mesenchymal transition (EndMT). LOXL2 knockdown in ECs results in decreased migration and sprouting, and concordantly, LOXL2 overexpression leads to an increase in migration and sprouting, independent of its catalytic activity. Furthermore, LOXL2 knockdown resulted in a reduced expression of EndMT markers, and inhibition of transforming growth factor-β (TGF-β)-mediated induction of EndMT. Interestingly, unlike in EMT, overexpression of LOXL2 alone is insufficient to induce EndMT. Further investigation revealed that LOXL2 expression regulates protein kinase B (PKB)/Akt and focal adhesion kinase (FAK) signaling, both pathways that have been implicated in the regulation of EMT. Altogether, our studies reveal a role for LOXL2 in angiogenesis through the modulation of EndMT in ECs, independent of its enzymatic crosslinking activity.
Keywords: angiogenesis; endothelial-to-mesenchymal transition; focal adhesion kinase; lysyl oxidase-like 2; protein kinase B.
© 2018 The Authors Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane.Blood. 2011 Oct 6;118(14):3979-89. doi: 10.1182/blood-2010-10-313296. Epub 2011 Aug 11. Blood. 2011. PMID: 21835952
-
Silencing of lysyl oxidase‑like 2 inhibits the migration, invasion and epithelial‑to‑mesenchymal transition of renal cell carcinoma cells through the Src/FAK signaling pathway.Int J Oncol. 2019 May;54(5):1676-1690. doi: 10.3892/ijo.2019.4726. Epub 2019 Feb 27. Int J Oncol. 2019. PMID: 30816490 Free PMC article.
-
Lysyl oxidase-like 2 (LOXL2) controls tumor-associated cell proliferation through the interaction with MARCKSL1.Cell Signal. 2014 Sep;26(9):1765-73. doi: 10.1016/j.cellsig.2014.05.010. Epub 2014 May 24. Cell Signal. 2014. PMID: 24863880
-
LOXL2 in cancer: regulation, downstream effectors and novel roles.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188435. doi: 10.1016/j.bbcan.2020.188435. Epub 2020 Sep 22. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32976981 Review.
-
Human lysyl oxidase-like 2.Bioorg Chem. 2014 Dec;57:231-241. doi: 10.1016/j.bioorg.2014.07.003. Epub 2014 Aug 1. Bioorg Chem. 2014. PMID: 25146937 Free PMC article. Review.
Cited by
-
Hypoxia Alters the Proteome Profile and Enhances the Angiogenic Potential of Dental Pulp Stem Cell-Derived Exosomes.Biomolecules. 2022 Apr 14;12(4):575. doi: 10.3390/biom12040575. Biomolecules. 2022. PMID: 35454164 Free PMC article.
-
LOXL2 Inhibitors and Breast Cancer Progression.Antioxidants (Basel). 2021 Feb 19;10(2):312. doi: 10.3390/antiox10020312. Antioxidants (Basel). 2021. PMID: 33669630 Free PMC article. Review.
-
The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications.Cancers (Basel). 2022 Feb 14;14(4):940. doi: 10.3390/cancers14040940. Cancers (Basel). 2022. PMID: 35205692 Free PMC article. Review.
-
A 3D-Predicted Structure of the Amine Oxidase Domain of Lysyl Oxidase-Like 2.Int J Mol Sci. 2022 Nov 2;23(21):13385. doi: 10.3390/ijms232113385. Int J Mol Sci. 2022. PMID: 36362176 Free PMC article.
-
Lysyl Oxidase Family Enzymes and Their Role in Tumor Progression.Int J Mol Sci. 2022 Jun 2;23(11):6249. doi: 10.3390/ijms23116249. Int J Mol Sci. 2022. PMID: 35682926 Free PMC article. Review.
References
-
- Ades, E. W. , Candal, F. J. , Swerlick, R. A. , George, V. G. , Summers, S. , Bosse, D. C. , & Lawley, T. J. (1992). HMEC‐1: Establishment of an immortalized human microvascular endothelial cell line. Journal of Investigative Dermatology, 99, 683–690. - PubMed
-
- van Balkom, B. W. M. , de Jong, O. G. , Smits, M. , Brummelman, J. , den Ouden, K. , de Bree, P. M. , … Verhaar, M. C. (2013). Endothelial cells require miR‐214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood, 121, 3997–4006. - PubMed
-
- Bignon, M. , Pichol‐Thievend, C. , Hardouin, J. , Malbouyres, M. , Brechot, N. , Nasciutti, L. , … Germain, S. (2011). Lysyl oxidase‐like protein‐2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood, 118, 3979–3989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

