Highly Diverse Hepatitis C Strains Detected in Sub-Saharan Africa Have Unknown Susceptibility to Direct-Acting Antiviral Treatments
- PMID: 30387174
- PMCID: PMC6492010
- DOI: 10.1002/hep.30342
Highly Diverse Hepatitis C Strains Detected in Sub-Saharan Africa Have Unknown Susceptibility to Direct-Acting Antiviral Treatments
Abstract
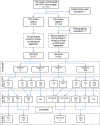
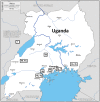
The global plan to eradicate hepatitis C virus (HCV) led by the World Health Organization outlines the use of highly effective direct-acting antiviral drugs (DAAs) to achieve elimination by 2030. Identifying individuals with active disease and investigation of the breadth of diversity of the virus in sub-Saharan Africa (SSA) is essential as genotypes in this region (where very few clinical trials have been carried out) are distinct from those found in other parts of the world. We undertook a population-based, nested case-control study in Uganda and obtained additional samples from the Democratic Republic of Congo (DRC) to estimate the prevalence of HCV, assess strategies for disease detection using serological and molecular techniques, and characterize genetic diversity of the virus. Using next-generation and Sanger sequencing, we aimed to identify strains circulating in East and Central Africa. A total of 7,751 Ugandan patients were initially screened for HCV, and 20 PCR-positive samples were obtained for sequencing. Serological assays were found to vary significantly in specificity for HCV. HCV strains detected in Uganda included genotype (g) 4k, g4p, g4q, and g4s and a newly identified unassigned g7 HCV strain. Two additional unassigned g7 strains were identified in patients originating from DRC (one partial and one full open reading frame sequence). These g4 and g7 strains contain nonstructural (ns) protein 3 and 5A polymorphisms associated with resistance to DAAs in other genotypes. Clinical studies are therefore indicated to investigate treatment response in infected patients. Conclusion: Although HCV prevalence and genotypes have been well characterized in patients in well-resourced countries, clinical trials are urgently required in SSA, where highly diverse g4 and g7 strains circulate.
© 2018 The Authors. Hepatology published by Wiley Periodicals, Inc. on behalf of American Association for the Study of Liver Diseases.
Figures

References
-
- World Health Organization . Guidelines for the Screening, Care and Treatment of Persons With Chronic HCV Infection. Geneva, Switzerland: World Health Organization; In press.
-
- World Health Organization . Global Hepatitis Report. Geneva, Switzerland: World Health Organization; 2017.
-
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus‐based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 2012;4:115ra1 10.1126/scitranslmed.3003155. http://stm.sciencemag.org - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12014/1/MRC_/Medical Research Council/United Kingdom
- G0901213-92157/MRC_/Medical Research Council/United Kingdom
- MC PC 16045/MRC_/Medical Research Council/United Kingdom
- MC_UU_12014/12/MRC_/Medical Research Council/United Kingdom
- G0901213/MRC_/Medical Research Council/United Kingdom
- 102789/Z/13/A/WT_/Wellcome Trust/United Kingdom
- MR/K013491/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_16045/MRC_/Medical Research Council/United Kingdom
- MC_UU_00027/1/MRC_/Medical Research Council/United Kingdom
- G0801566/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

