Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate
- PMID: 30387335
- PMCID: PMC6238115
- DOI: 10.1631/jzus.B1800132
Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate
Abstract
Objective: Keloids are exuberant cutaneous scars that form due to abnormal growth of fibrous tissue following an injury. The primary aim of this study was to assess the efficacy and mechanism of hyperbaric oxygen therapy (HBOT) to reduce the keloid recurrence rate after surgical excision and radiotherapy.
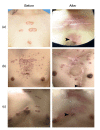
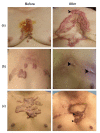
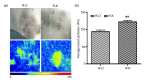
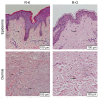
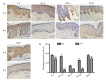
Methods: (1) A total of 240 patients were randomly divided into two groups. Patients in the HBOT group (O group) received HBOT after surgical excision and radiotherapy. Patients in the other group were treated with only surgical excision and radiotherapy (K group). (2) Scar tissue from recurrent patients was collected after a second operation. Hematoxylin and eosin (H&E) staining was used to observe keloid morphology. Certain inflammatory factors (interleukin-6 (IL-6), hypoxia-inducible factor-1α (HIF-1α), tumor necrosis factor-α (TNF-α), nuclear factor κB (NF-κB), and vascular endothelial growth factor (VEGF)) were measured using immunohistochemical staining.
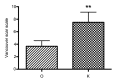
Results: (1) The recurrence rate of the O group (5.97%) was significantly lower than that of the K group (14.15%), P<0.05. Moreover, patients in the O group reported greater satisfaction than those in the K group (P<0.05). (2) Compared with the recurrent scar tissue of the K group, the expression levels of the inflammatory factors were lower in the recurrent scar tissue of the O group.
Conclusions: Adjunctive HBOT effectively reduces the keloid recurrence rate after surgical excision and radiotherapy by improving the oxygen level of the tissue and alleviating the inflammatory process.
Keywords: Keloid; Hyperbaric oxygen therapy; Surgical excision; Radiotherapy; Recurrence rate.
Conflict of interest statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this article.
Figures
References
-
- Alexandrescu D, Fabi S, Yeh LC, et al. Comparative results in treatment of keloids with intralesional 5-FU/kenalog, 5-FU/verapamil, enalapril alone, verapamil alone, and laser: a case report and review of the literature. J Drugs Dermatol. 2016;15(11):1442–1447. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

