Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study
- PMID: 30387366
- PMCID: PMC6371765
- DOI: 10.1289/EHP3953
Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study
Abstract
Background: Previous work determined that early life exposure to low-dose Bisphenol A (BPA) increased rat prostate cancer risk with aging. Herein, we report on prostate-specific results from CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity), which aims to resolve uncertainties regarding BPA toxicity.
Objectives: We sought to a) reassess whether a range of BPA exposures drives prostate pathology and/or alters prostatic susceptibility to hormonal carcinogenesis, and b) test whether chronic low-dose BPA targets prostate epithelial stem and progenitor cells.
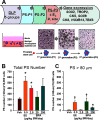
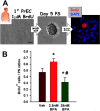
Methods: Sprague-Dawley rats were gavaged daily with vehicle, ethinyl estradiol (EE) or [Formula: see text] BPA/kg-BW during development or chronically, and prostate pathology was assessed at one year. One developmentally exposed cohort was given testosterone plus estradiol ([Formula: see text]) implants at day 90 to promote carcinogenesis with aging. Epithelial stem and progenitor cells were isolated by prostasphere (PS) culture from dorsolateral prostates (DLP) of rats continuously exposed for six months to [Formula: see text] BPA/kg-BW. Gene expression was analyzed by quantitative real time reverse transcription polymerase chain reaction (qRT-PCR).
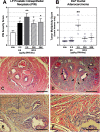
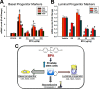
Results: Exposure to BPA alone at any dose did not drive prostate pathology. However, rats treated with EE, 2.5, 250, or [Formula: see text] BPA/kg-BW plus [Formula: see text] showed greater severity of lateral prostate intraepithelial neoplasia (PIN), and DLP ductal adenocarcinoma multiplicity was markedly elevated in tumor-bearing rats exposed to [Formula: see text]-BW. DLP stem cells, assessed by PS number, doubled with chronic EE and [Formula: see text] exposures. PS size, reflecting progenitor cell proliferation, was greater at 25 and [Formula: see text] BPA doses, which also shifted lineage commitment toward basal progenitors while reducing luminal progenitor cells.
Conclusions: Together, these results confirm and extend previous evidence using a rat model and human prostate epithelial cells that low-dose BPA augments prostate cancer susceptibility and alters adult prostate stem cell homeostasis. Therefore, we propose that BPA exposures may contribute to the increased carcinogenic risk in humans that occurs with aging. https://doi.org/10.1289/EHP3953.
Figures

References
-
- Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB. 2016. Impact of low dose oral exposure to bisphenol A (BPA) on the neonatal rat hypothalamic and hippocampal transcriptome: a CLARITY-BPA consortium study. Endocrinology 157(10):3856–3872, PMID: 27571134, 10.1210/en.2016-1339. - DOI - PMC - PubMed
-
- Aungst J, Twaroski ML. 2009. “Bisphenol A (CAS RN. 80-05-7): Review of low dose studies memorandum.” Washington, DC:U.S. Food and Drug Administration, Document 1 FDA-2010-N-0100-0001; pg 1–86, https://www.regulations.gov/document?D=FDA-2010-N-0100-0006 [accessed April 2018].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
