Reported willingness to participate in a hypothetical HIV vaccine trial and its translation to actual participation among healthy adults-Experience from Kenya
- PMID: 30388145
- PMCID: PMC6214541
- DOI: 10.1371/journal.pone.0206656
Reported willingness to participate in a hypothetical HIV vaccine trial and its translation to actual participation among healthy adults-Experience from Kenya
Abstract
Objective: To evaluate initial reported willingness to participate in a hypothetical HIV vaccine clinical trial and actual participation of volunteers in a longitudinal observational study.
Methods: We recruited HIV negative male and female volunteers aged 18-45 years into a longitudinal observational study at KAVI-ICR Kangemi in Kenya, to serve as a pool from which to draw participants into a phase I HIV vaccine clinical trial. A structured questionnaire was used to collect information regarding willingness to join a HIV vaccine clinical trial in the future. Study follow-up visits were every 6 months.
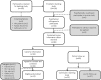
Results: A total of 105 participants were screened and 100 (M46:F54) were enrolled into the observational study. Ninety- four per cent of those enrolled expressed willingness to participate in a future HIV vaccine trial. Altruism and desire to learn the body's response to the vaccine were the most motivating factors at 40% and 25% respectively. At the onset of a 40-person phase I HIV vaccine trial, 86 observational study participants who had previously expressed willingness to participate were contacted but only 26 (30%) came for information. All 26 consented to participate and after screening for eligibility, 24 were eligible. Of the 24, 15 were enrolled. These numbers were not adequate; hence the vaccine trial employed other recruitment methods to meet the deficit.
Conclusion: Observational "pools" of cohorts may not provide adequate number of participants into vaccine clinical trials even if they report willingness; therefore supplementary recruitment methods such as direct community recruitment, passive approach, and snowballing need to be in place.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Motivators of enrolment in HIV vaccine trials: a review of HIV vaccine preparedness studies.AIDS Care. 2011 Nov;23(11):1430-47. doi: 10.1080/09540121.2011.555750. Epub 2011 Jul 4. AIDS Care. 2011. PMID: 21722022
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antidepressants for depression in adults with HIV infection.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD008525. doi: 10.1002/14651858.CD008525.pub3. Cochrane Database Syst Rev. 2018. PMID: 29355886 Free PMC article.
Cited by
-
Willingness of female sex workers in Kampala, Uganda to participate in future HIV vaccine trials: a case control study.BMC Public Health. 2020 Nov 25;20(1):1789. doi: 10.1186/s12889-020-09932-7. BMC Public Health. 2020. PMID: 33239018 Free PMC article.
-
Community Health Volunteers' experiences of implementing COVID-19 vaccine education and promotion in Kenya: a qualitative descriptive study.Front Public Health. 2024 Jul 10;12:1406959. doi: 10.3389/fpubh.2024.1406959. eCollection 2024. Front Public Health. 2024. PMID: 39050596 Free PMC article.
-
Understanding the Acceptability of Broadly Neutralizing Antibodies for HIV Prevention Among At-Risk Populations and Feasibility Considerations for Product Introduction in India: Protocol for a Qualitative Study.JMIR Res Protoc. 2024 Feb 7;13:e47700. doi: 10.2196/47700. JMIR Res Protoc. 2024. PMID: 38324364 Free PMC article.
-
Variations in racial and ethnic groups' trust in researchers associated with willingness to participate in research.Humanit Soc Sci Commun. 2023;10:466. doi: 10.1057/s41599-023-01960-z. Epub 2023 Aug 2. Humanit Soc Sci Commun. 2023. PMID: 38650745 Free PMC article.
-
Willingness to participate in COVID-19 vaccine trials; a survey among a population of healthcare workers in Uganda.PLoS One. 2021 May 27;16(5):e0251992. doi: 10.1371/journal.pone.0251992. eCollection 2021. PLoS One. 2021. PMID: 34043693 Free PMC article.
References
-
- Global AIDS Update 2016—global-AIDS-update-2016_en.pdf [Internet]. [cited 2017 May 20]. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update...
-
- Brandt D. Factors associated with young adults’ reported intention of willingness to participate in clinical research. Theses Diss [Internet]. 2013 Jan 1; http://ir.uiowa.edu/etd/1552
-
- Carter RE, Sonne SC, Brady KT. Practical considerations for estimating clinical trial accrual periods: application to a multi-center effectiveness study. BMC Med Res Methodol [Internet]. 2005. December [cited 2017 Mar 12];5(1). http://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-5-11 - DOI - PMC - PubMed
-
- Baines CJ, Christen A, Simard A, Wall C, Dean D, Duncan L, et al. The National Breast Screening Study: pre-recruitment sources of awareness in participants. Can J Public Health Rev Can Sante Publique. 1989. June;80(3):221–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical