Validation of near infrared fluorescence (NIRF) probes in vivo with dual laser NIRF endoscope
- PMID: 30388158
- PMCID: PMC6214553
- DOI: 10.1371/journal.pone.0206568
Validation of near infrared fluorescence (NIRF) probes in vivo with dual laser NIRF endoscope
Erratum in
-
Correction: Validation of near infrared fluorescence (NIRF) probes in vivo with dual laser NIRF endoscope.PLoS One. 2019 Jan 7;14(1):e0210677. doi: 10.1371/journal.pone.0210677. eCollection 2019. PLoS One. 2019. PMID: 30615682 Free PMC article.
Abstract
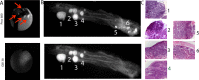
Purpose: The development of NIRF cathepsin activity probes offered the ability to visualize tumor associated tumor reaction and act as a surrogate marker to delineate the dysplastic lesions. One major type is a NIRF substrate of cathepsins (SBP), which is involved in catalytic way to produce high levels of fluorescence emission. The other major type (ABP) reacts with active cathepsins in stoichiometric manner since they bind covalently with their active center. Little is known about the sensitivity and the specificity of the NIRF probes to detect autochthonous developed dysplastic lesions. Dual laser NIRF endoscope provides a good tool to determine the efficiency of various NIRF probes in vivo in the same lesions.
Experimental design: In the current study, we validated both types of NIRF probes by using the dual laser NIRF endoscope to detect lesions colon cancer mouse model (TS4Cre/cAPC +/lox).
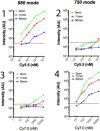
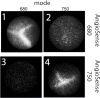
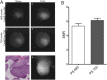
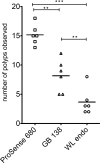
Results: The dual laser NIRF endoscope is emitting equal power with both lasers. It can detect with the same efficiency in 680 mode, as well as, 750 mode when NIFR probes of the same scaffold in vivo. When SBP and ABP were used, our results showed both probes are efficient enough to detect large polyps but small dysplastic lesions could not efficiently imaged with the ABP.
Conclusions: The dual laser NIRF endoscope is a powerful tool to validate probes. The probes that react catalytically with the active center of cathepsins are more efficient than the ones that react stoichiometrically in detecting small lesions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Choi PM, Nugent FW, Schoetz DJ, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. YGAST. 1993;105: 418–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

