DNA vaccine priming for seasonal influenza vaccine in children and adolescents 6 to 17 years of age: A phase 1 randomized clinical trial
- PMID: 30388160
- PMCID: PMC6214651
- DOI: 10.1371/journal.pone.0206837
DNA vaccine priming for seasonal influenza vaccine in children and adolescents 6 to 17 years of age: A phase 1 randomized clinical trial
Abstract
Background: Children are susceptible to severe influenza infections and facilitate community transmission. One potential strategy to improve vaccine immunogenicity in children against seasonal influenza involves a trivalent hemagglutinin DNA prime-trivalent inactivated influenza vaccine (IIV3) boost regimen.
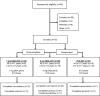
Methods: Sites enrolled adolescents, followed by younger children, to receive DNA prime (1 mg or 4 mg) intramuscularly by needle-free jet injector (Biojector), followed by split virus 2012/13 seasonal IIV3 boost by needle and syringe approximately 18 weeks later. A comparator group received IIV3 prime and boost at similar intervals. Primary study objectives included evaluation of the safety and tolerability of the vaccine regimens, with secondary objectives of measuring antibody responses at four weeks post boost by hemagglutination inhibition (HAI) and neutralization assays.
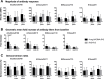
Results: Seventy-five children ≥6 to ≤17 years old enrolled. Local reactogenicity was higher after DNA prime compared to IIV3 prime (p<0.001 for pain/tenderness, redness, or swelling), but symptoms were mild to moderate in severity. Systemic reactogenicity was similar between vaccines. Overall, antibody responses were similar among groups, although HAI antibodies revealed a trend towards higher responses following 4 mg DNA-IIV3 compared to IIV3-IIV3. The fold increase of HAI antibodies to A/California/07/2009 [A(H1N1)pdm09] was significantly greater following 4 mg DNA-IIV3 (10.12 fold, 5.60-18.27 95%CI) compared to IIV3-IIV3 (3.86 fold, 2.32-6.44 95%CI). Similar neutralizing titers were observed between regimens, with a trend towards increased response frequencies in 4 mg DNA-IIV3. However, significant differences in fold increase, reported as geometric mean fold ratios, were detected against the H1N1 viruses within the neutralization panel: A/New Caledonia/20/1999 (1.41 fold, 1.10-1.81 95%CI) and A/South Carolina/1/1918 (1.55 fold, 1.27-1.89 95%CI).
Conclusions: In this first pediatric DNA vaccine study conducted in the U.S., the DNA prime-IIV3 boost regimen was safe and well tolerated. In children, the 4 mg DNA-IIV3 regimen resulted in antibody responses comparable to the IIV3-IIV3 regimen.
Conflict of interest statement
I have read the journal's policy and the authors of the manuscript have the following competing interests: this clinical study was conducted with funding and support by the National Institute of Allergy and Infectious Diseases (NIAID) Intramural Research program, using resources provided by the American Recovery and Reinvestment Act of 2009 (Recovery Act), and contract #HHSN272201000049I awarded to the EMMES Corporation (AB, JM, EA, DB, BC, HK, PS, PW). AB and JM are salaried employees of the EMMES Corporation. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Fowlkes A, Steffens A, Temte J, Di Lonardo S, McHugh L, Martin K, et al. Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009–13. Lancet Respir Med. 2015;3(9):709–18. 10.1016/S2213-2600(15)00278-7 . - DOI - PMC - PubMed
-
- Cauchemez S, Bhattarai A, Marchbanks TL, Fagan RP, Ostroff S, Ferguson NM, et al. Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Natl Acad Sci U S A. 2011;108(7):2825–30. 10.1073/pnas.1008895108 ; PubMed Central PMCID: PMCPMC3041067. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

