c-Myc is a regulator of the PKD1 gene and PC1-induced pathogenesis
- PMID: 30388220
- PMCID: PMC6381314
- DOI: 10.1093/hmg/ddy379
c-Myc is a regulator of the PKD1 gene and PC1-induced pathogenesis
Abstract
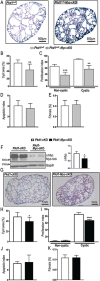
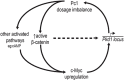
Autosomal dominant polycystic kidney disease (ADPKD) is among the most common monogenic disorders mainly associated with PKD1/PC1 mutations. We show herein that renal regulation in Pc1 dosage-reduced and -increased mouse models converge toward stimulation of c-Myc expression along with β-catenin, delineating c-Myc as a key Pkd1 node in cystogenesis. Enhanced renal c-Myc-induced ADPKD in SBM transgenic mice lead conversely to striking upregulation of Pkd1/Pc1 expression and β-catenin activation, lending credence for reciprocal crosstalk between c-Myc and Pc1. In adult SBM kidneys, c-Myc is strongly enriched on Pkd1 promoter with RNA pol II, consistent with Pkd1 upregulation during cystogenesis. Similar c-Myc direct binding at birth uncovers an equivalent role on Pkd1 regulation during renal developmental program. Concurrent with enriched c-Myc binding, recruitment of active chromatin modifying co-factors by c-Myc at the Pkd1 regulatory region probably opens chromatin to stimulate transcription. A similar transcriptional activation by c-Myc is also likely operant on endogenous human PKD1 gene from our transactivation analysis in response to human c-MYC upregulation. Genetic ablation of c-Myc in Pc1-reduced and -increased mouse models significantly attenuates cyst growth, proliferation and PKD progression. Our study determined a dual role for c-Myc, as a major contributor in Pc1-induced cystogenesis and in a feed-forward regulatory Pkd1-c-Myc loop mechanism that may also prevail in human ADPKD.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
TAZ/Wnt-β-catenin/c-MYC axis regulates cystogenesis in polycystic kidney disease.Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):29001-29012. doi: 10.1073/pnas.2009334117. Epub 2020 Oct 29. Proc Natl Acad Sci U S A. 2020. PMID: 33122431 Free PMC article.
-
The master regulators Myc and p53 cellular signaling and functions in polycystic kidney disease.Cell Signal. 2020 Jul;71:109594. doi: 10.1016/j.cellsig.2020.109594. Epub 2020 Mar 4. Cell Signal. 2020. PMID: 32145315 Review.
-
Progressive development of polycystic kidney disease in the mouse model expressing Pkd1 extracellular domain.Hum Mol Genet. 2013 Jun 15;22(12):2361-75. doi: 10.1093/hmg/ddt081. Epub 2013 Feb 25. Hum Mol Genet. 2013. PMID: 23439951
-
Cux1 promotes cell proliferation and polycystic kidney disease progression in an ADPKD mouse model.Am J Physiol Renal Physiol. 2017 Oct 1;313(4):F1050-F1059. doi: 10.1152/ajprenal.00380.2016. Epub 2017 Jul 12. Am J Physiol Renal Physiol. 2017. PMID: 28701314 Free PMC article.
-
Genetic Mechanisms of ADPKD.Adv Exp Med Biol. 2016;933:13-22. doi: 10.1007/978-981-10-2041-4_2. Adv Exp Med Biol. 2016. PMID: 27730431 Review.
Cited by
-
c-Jun N-terminal kinase (JNK) signaling contributes to cystic burden in polycystic kidney disease.PLoS Genet. 2021 Dec 28;17(12):e1009711. doi: 10.1371/journal.pgen.1009711. eCollection 2021 Dec. PLoS Genet. 2021. PMID: 34962918 Free PMC article.
-
MYC: there is more to it than cancer.Front Cell Dev Biol. 2024 Mar 6;12:1342872. doi: 10.3389/fcell.2024.1342872. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38510176 Free PMC article. Review.
-
Cystin genetic variants cause autosomal recessive polycystic kidney disease associated with altered Myc expression.Sci Rep. 2021 Sep 14;11(1):18274. doi: 10.1038/s41598-021-97046-4. Sci Rep. 2021. PMID: 34521872 Free PMC article.
-
SOX9-dependent fibrosis drives renal function in nephronophthisis.EMBO Mol Med. 2025 Jun;17(6):1238-1258. doi: 10.1038/s44321-025-00233-3. Epub 2025 Apr 10. EMBO Mol Med. 2025. PMID: 40211043 Free PMC article.
-
H3K14 hyperacetylation‑mediated c‑Myc binding to the miR‑30a‑5p gene promoter under hypoxia postconditioning protects senescent cardiomyocytes from hypoxia/reoxygenation injury.Mol Med Rep. 2021 Jun;23(6):468. doi: 10.3892/mmr.2021.12107. Epub 2021 Apr 21. Mol Med Rep. 2021. PMID: 33880587 Free PMC article.
References
-
- Lanoix J., D'Agati V., Szabolcs M. and Trudel M. (1996) Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene, 13, 1153–1160. - PubMed
-
- Ward C.J., Turley H., Ong A.C.M., Comley M., Biddolph S., Chetty R., Ratcliffe P.J., Gatter K. and Harris P.C. (1996) Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult and polycystic kidney. Proc. Natl. Acad. Sci. U. S. A., 93, 1524–1528. - PMC - PubMed
-
- Van Adelsberg J., Chamberlain S. and D'Agati V. (1997) Polycystin expression is temporally and spatially regulated during renal development. Am. J. Physiol., 272, F602–F609. - PubMed
-
- Ibraghimov-Beskrovnaya O., Dackowski W.R., Foggensteiner L., Coleman N., Thiru S., Petry L.R., Burn T.C., Connors T.D., Van Raay T., Bradley J. et al. (1997) Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc. Natl. Acad. Sci. U. S. A., 94, 6397–6402. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

