A Dual-Function Antibiotic-Transporter Conjugate Exhibits Superior Activity in Sterilizing MRSA Biofilms and Killing Persister Cells
- PMID: 30388366
- PMCID: PMC6430714
- DOI: 10.1021/jacs.8b08711
A Dual-Function Antibiotic-Transporter Conjugate Exhibits Superior Activity in Sterilizing MRSA Biofilms and Killing Persister Cells
Abstract
New strategies are urgently needed to target MRSA, a major global health problem and the leading cause of mortality from antibiotic-resistant infections in many countries. Here, we report a general approach to this problem exemplified by the design and synthesis of a vancomycin-d-octaarginine conjugate (V-r8) and investigation of its efficacy in addressing antibiotic-insensitive bacterial populations. V-r8 eradicated MRSA biofilm and persister cells in vitro, outperforming vancomycin by orders of magnitude. It also eliminated 97% of biofilm-associated MRSA in a murine wound infection model and displayed no acute dermal toxicity. This new dual-function conjugate displays enhanced cellular accumulation and membrane perturbation as compared to vancomycin. Based on its rapid and potent activity against biofilm and persister cells, V-r8 is a promising agent against clinical MRSA infections.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


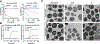


References
-
- Klein EY; Mojica N; Jiang W; Cosgrove SE; Septimus E; Morgan DJ; Laxminarayan R Trends in Methicillin-Resistant Staphylococcus Aureus Hospitalizations in the United States, 2010–2014. Clin. Infect. Dis 2017, 65, 1921–1923. - PubMed
-
- Stefani S; Chung DR; Lindsay JA; Friedrich AW; Kearns AM; Westh H; MacKenzie FM Meticillin-Resistant Staphylococcus Aureus (MRSA): Global Epidemiology and Harmonisation of Typing Methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. - PubMed
-
- Tattevin P; Schwartz BS; Graber CJ; Volinski J; Bhukhen A; Bhukhen A; Mai TT; Vo NH; Dang DN; Phan TH; Basuino L; Perdreau-Remington F; Chambers HF; Diep BA Concurrent Epidemics of Skin and Soft Tissue Infection and Bloodstream Infection Due to Community-Associated Methicillin-Resistant Staphylococcus Aureus. Clin. Infect. Dis 2012, 55, 781–788. - PMC - PubMed
-
- Fisher RA; Gollan B; Helaine S Persistent Bacterial Infections and Persister Cells. Nat. Rev. Microbiol 2017, 15, 453–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

