Cationic Oligospermine-Oligonucleotide Conjugates Provide Carrier-free Splice Switching in Monolayer Cells and Spheroids
- PMID: 30388622
- PMCID: PMC6205332
- DOI: 10.1016/j.omtn.2018.09.027
Cationic Oligospermine-Oligonucleotide Conjugates Provide Carrier-free Splice Switching in Monolayer Cells and Spheroids
Abstract
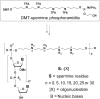
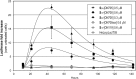
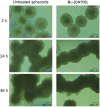
We report the evaluation of 18-mer 2'-O-methyl-modified ribose oligonucleotides with a full-length phosphorothioate backbone chemically conjugated at the 5' end to the oligospermine units (Sn-: n = 5, 15, 20, 25, and 30 [number of spermine units]) as splice switching oligonucleotides (SSOs). These conjugates contain, in their structure, covalently linked oligocation moieties, making them capable of penetrating cells without transfection vector. In cell culture, we observed efficient cytoplasmic and nuclear delivery of fluorescein-labeled S20-SSO by fluorescent microscopy. The SSO conjugates containing more than 15 spermine units induced significant carrier-free exon skipping at nanomolar concentration in the absence and in the presence of serum. With an increasing number of spermine units, the conjugates became slightly toxic but more active. Advantages of these molecules were particularly demonstrated in three-dimensional (3D) cell culture (multicellular tumor spheroids [MCTSs]) that mimics living tissues. Whereas vector-complexed SSOs displayed a drastically reduced splice switching in MCTS compared with the assay in monolayer culture, an efficient exon skipping without significant toxicity was observed with oligospermine-grafted SSOs (S15- and S20-SSOs) transfected without vector. It was shown, by flow cytometry and confocal microscopy, that the fluorescein-labeled S20-SSO was freely diffusing and penetrating the innermost cells of MCTS, whereas the vector-complexed SSO penetrated only the cells of the spheroid's outer layer.
Keywords: MCTS; SSO; carrier-free oligonucleotide delivery; cationic oligonucleotide-oligospermine conjugate; exon skipping; multicellular tumor spheroids; splice switching oligonucleotides.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

