Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress
- PMID: 30388684
- PMCID: PMC6216086
- DOI: 10.1016/j.redox.2018.10.019
Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress
Erratum in
-
Corrigendum to "Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress" [Redox Biol. 20 (2019) 296-306].Redox Biol. 2019 Sep;26:101193. doi: 10.1016/j.redox.2019.101193. Epub 2019 Apr 29. Redox Biol. 2019. PMID: 31043340 Free PMC article. No abstract available.
Abstract
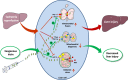
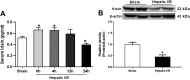
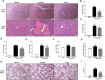
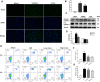
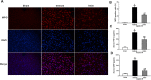
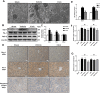
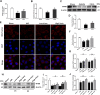
Current management of liver ischemia-reperfusion (I/R) injury is mainly based on supportive care and no specific treatment is available. Irisin, a recently identified hormone, plays pivotal roles in energy expenditure and oxidative metabolism; however, it remains unknown whether irisin has any protective effects on hepatic I/R injury. In this study, we found that serum and liver irisin levels were markedly decreased at 24 h after hepatic I/R. Treatment with exogenous irisin improved liver function, reduced liver necrosis and cell apoptosis, and relieved inflammatory response after hepatic I/R. Meanwhile, exogenous irisin markedly inhibited mitochondrial fission related protein dynamin related protein 1 (drp-1) and fission 1 (Fis-1) expression in hepatic I/R. Additionally, treatment with exogenous irisin increased mitochondrial content and increased mitochondrial biogenesis related peroxisome proliferative activated receptor-γ (PPARγ) co-activator 1α (PGC-1α) and mitochondrial transcription factor (TFAM) expression. Furthermore, irisin decreased oxidative stress by upregulating uncoupling proteins (UCP) 2 expression in hepatic I/R. The results reveal that treatment with exogenous irisin alleviated hepatic I/R injury by restraining mitochondrial fission, promoting mitochondrial biogenesis and relieving oxidative stress. Irisin treatment appears to be a novel and promising therapeutic approach for hepatic I/R injury.
Keywords: Hepatic I/R; Irisin; Mitochondrial homeostasis; Oxidative stress.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

