Familial Hypercholesterolemia: The Most Frequent Cholesterol Metabolism Disorder Caused Disease
- PMID: 30388787
- PMCID: PMC6275065
- DOI: 10.3390/ijms19113426
Familial Hypercholesterolemia: The Most Frequent Cholesterol Metabolism Disorder Caused Disease
Abstract
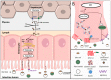
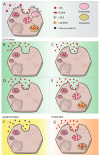
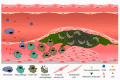
Cholesterol is an essential component of cell barrier formation and signaling transduction involved in many essential physiologic processes. For this reason, cholesterol metabolism must be tightly controlled. Cell cholesterol is mainly acquired from two sources: Dietary cholesterol, which is absorbed in the intestine and, intracellularly synthesized cholesterol that is mainly synthesized in the liver. Once acquired, both are delivered to peripheral tissues in a lipoprotein dependent mechanism. Malfunctioning of cholesterol metabolism is caused by multiple hereditary diseases, including Familial Hypercholesterolemia, Sitosterolemia Type C and Niemann-Pick Type C1. Of these, familial hypercholesterolemia (FH) is a common inherited autosomal co-dominant disorder characterized by high plasma cholesterol levels. Its frequency is estimated to be 1:200 and, if untreated, increases the risk of premature cardiovascular disease. This review aims to summarize the current knowledge on cholesterol metabolism and the relation of FH to cholesterol homeostasis with special focus on the genetics, diagnosis and treatment.
Keywords: cholesterol; familial hypercholesterolemia; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cherezov V., Rosenbaum D.M., Hanson M.A., Rasmussen S.G.F., Thian F.S., Kobilka T.S., Choi H.-J., Kuhn P., Weis W.I., Kobilka B.K., et al. High-Resolution Crystal Structure of an Engineered Human 2-Adrenergic G Protein-Coupled Receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

