Fludarabine with a higher versus lower dose of myeloablative timed-sequential busulfan in older patients and patients with comorbidities: an open-label, non-stratified, randomised phase 2 trial
- PMID: 30389035
- PMCID: PMC6317874
- DOI: 10.1016/S2352-3026(18)30156-X
Fludarabine with a higher versus lower dose of myeloablative timed-sequential busulfan in older patients and patients with comorbidities: an open-label, non-stratified, randomised phase 2 trial
Abstract
Background: Haemopoietic stem-cell transplantation (HCT) conditioning regimens that can reduce risk of relapse without increasing non-relapse mortality are needed. We aimed to test the safety of timed-sequential delivery of low-dose versus high-dose myeloablative busulfan in older patients and patients with comorbidities.
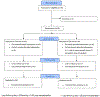
Methods: This non-stratified, open-label, randomised phase 2 trial was done at The University of Texas MD Anderson Cancer Center (Houston, TX, USA). Patients with haematological cancers aged between 5 and 75 years were eligible to participate in the study. Patients who had HIV or uncontrollable infections were excluded. Eligible patients were randomly assigned (1:1 by a computer-generated programme in block sizes of four) to receive a total intravenous busulfan dose to achieve an area under the curve of 16 000 μmol/min (16K group) or 20 000 μmol/min (20K group) on the basis of pharmacokinetic analysis, plus intravenous fludarabine 40 mg/m2 for 4 days. The investigators and the research nurses were masked to the block size to conceal allocation. The primary outcome was day 100 non-relapse mortality. All analyses were by modified intention to treat, including only patients who received at least one dose of the study drug. No interim analyses were planned and accrual is complete. This study is registered with ClinicalTrials.gov, number NCT01572662.
Findings: Between April 18, 2012, and Dec 9, 2015, 98 patients were enrolled. 49 patients were randomly assigned to the 16K group and 49 to the 20K group, one of which was removed from the study before starting the intervention. Median age was 60 years (IQR 54-67). 50 (52%) patients had an HCT-specific comorbidity index score of 3 or more, and 41 (42%) had a high or very high Disease Risk Index score. Day 100 non-relapse mortality was 4% (95% CI 0-10) in the 16K group and 6% (0-13) in the 20K group (p=0·65). Infection was the most common grade 3-5 toxicity in both the 20K group (25 [52%] of 48 patients) and the 16K group (24 [49%] of 49 participants). Mucositis (nine [19%] of 48 patients vs three [6%] of 49 patients), idiopathic pneumonia syndrome (nine [19%] of 48 patients vs two [4%] of 49 patients), and culture-negative neutropenic fever (16 [33%] of 48 patients vs eight [16%] of 49 patients) were more common in the 20K group than in the 16K group.
Interpretation: Myeloablative doses of busulfan administered in a timed-sequential manner with fludarabine is associated with low non-relapse mortality in older patients and patients with comorbidities. Additional studies are required to show whether this approach can reduce the risk of relapse.
Funding: Cancer Center Support Grant (US National Cancer Institute, National Institutes of Health).
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

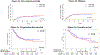
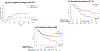
References
-
- Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–44. - PubMed
-
- Lioure B, Bene MC, Pigneux A, Huynh A, Chevallier P, Fegueux N, et al. Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: long-term results of a prospective GOELAMS study. Blood. 2012;119(12):2943–8. - PubMed
-
- Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7. - PubMed
-
- Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

