Mitochondrial glycerol 3-phosphate dehydrogenase promotes skeletal muscle regeneration
- PMID: 30389681
- PMCID: PMC6284384
- DOI: 10.15252/emmm.201809390
Mitochondrial glycerol 3-phosphate dehydrogenase promotes skeletal muscle regeneration
Abstract
While adult mammalian skeletal muscle is stable due to its post-mitotic nature, muscle regeneration is still essential throughout life for maintaining functional fitness. During certain diseases, such as the modern pandemics of obesity and diabetes, the regeneration process becomes impaired, which leads to the loss of muscle function and contributes to the global burden of these diseases. However, the underlying mechanisms of the impairment are not well defined. Here, we identify mGPDH as a critical regulator of skeletal muscle regeneration. Specifically, it regulates myogenic markers and myoblast differentiation by controlling mitochondrial biogenesis via CaMKKβ/AMPK. mGPDH-/- attenuated skeletal muscle regeneration in vitro and in vivo, while mGPDH overexpression ameliorated dystrophic pathology in mdx mice. Moreover, in patients and animal models of obesity and diabetes, mGPDH expression in skeletal muscle was reduced, further suggesting a direct correlation between its abundance and muscular regeneration capability. Rescuing mGPDH expression in obese and diabetic mice led to a significant improvement in their muscle regeneration. Our study provides a potential therapeutic target for skeletal muscle regeneration impairment during obesity and diabetes.
Keywords: diabetes; mGPDH; obesity; skeletal muscle regeneration.
© 2018 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
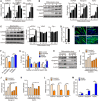
- A, B
qRT–PCR (A) and immunoblot (B) of mGPDH, myogenin, and myosin heavy chain (MyHC) levels during C2C12 myocyte differentiation. Quantification represents the levels of the indicated protein normalized to β‐actin.
- C
Immunoblot of mGPDH, voltage‐dependent anion channel (VDAC), and cytochrome c (Cyt C) levels in mitochondrial lysate during C2C12 myocyte differentiation. Quantification represents the levels of the indicated protein normalized to COX IV.
- D
Activity assay of mGPDH at days 0 and 7 after C2C12 myocyte differentiation.
- E–G
Representative images of MyHC immunofluorescence (E) of C2C12 myocyte transfected with the siRNA or the overexpression plasmid for mGPDH; the fusion index (F) and the distribution of nuclei per myotube (G) were calculated at day 5 after differentiation.
- H–K
Immunoblot of mGPDH, myogenin, and MyHC in C2C12 myocytes transfected with siRNA targeting mGPDH. Quantification (I–K) represents the levels of the indicated protein normalized to β‐actin at the indicated day after differentiation.
- L, M
qRT–PCR analysis of mGPDH, myogenin, and MyHC in C2C12 myocytes transfected with the siRNA or the overexpression plasmid for mGPDH at day 4 after differentiation.
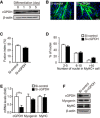
- A
cGPDH expression during C2C12 myocyte differentiation.
- B–D
Representative images of MyHC immunofluorescence (B) of C2C12 myocytes transfected with siRNA targeting cGPDH; the fusion index (C) and the distribution of nuclei per myotube (D) were calculated.
- E, F
qRT–PCR (E) and Western blot analysis (F) of myogenin and MyHC in C2C12 myocytes transfected with siRNA targeting cGPDH.
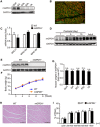
- A
Immunoblot of mGPDH in the quadriceps (QUA), gastrocnemius (GA), soleus (SOL), extensor digitorum longus (EDL), and tibialis anterior (TA) muscles of 8‐week‐old C57BL/6J mice.
- B
Immunofluorescence showing localization of mGPDH with fiber type marker MHC IIb on cryosections from uninjured GA muscle of 8‐week‐old C57BL/6J mice.
- C
qRT–PCR analyses of the indicated fiber type markers (MHC I, IIa, and IIb) in the uninjured GA muscles of 8‐week‐old WT and mGPDH−/− mice.
- D
Immunoblot of mGPDH in C57BL/6J mouse skeletal muscle at postnatal days 1, 5, and 10 and 8 weeks.
- E
Immunoblot of mGPDH in the GA muscle of 8‐week WT and mGPDH−/− mice.
- F
Body weight of WT and mGPDH−/− mice at the indicated week of age.
- G
Muscle weight of the indicated 8‐week‐old mGPDH−/− mice normalized to WT.
- H, I
Hematoxylin–eosin (H&E) staining (H) and average myofiber cross‐sectional area (CSA) (I) in the GA muscle of 8‐week‐old WT and mGPDH−/− mice.

- A, B
qRT–PCR (A) and immunoblot (B) of mGPDH, myogenin, and developmental myosin heavy chain (myh8, myl4, and myh3) in gastrocnemius (GA) muscle from C57BL/6J mice at the indicated day after CTX intramuscular injection.
- C
Activity assay of mGPDH in GA muscle from C57BL/6J mice at days 0 and 7 after CTX injection.
- D–G
Representative images of the H&E staining (arrowhead, necrotic myofibers; asterisks, regenerating fibers) (D), distribution of the fiber cross‐sectional area (CSA) (E), percentage of myofibers with central nuclei (F), and immunofluorescence staining of desmin (green) (G) in GA muscle from WT and mGPDH−/− mice at day 7 post‐CTX injection.
- H, I
Muscle weight (H) and trichrome staining (I) in GA muscle from WT and mGPDH−/− mice at day 14 post‐CTX injection. Quantification represents the fibrotic areas.
- J, K
qRT–PCR (J) and immunoblot (K) for mGPDH, myogenin, and myh3 in GA muscle from WT and mGPDH−/− mice at day 7 post‐CTX injection.
- L–Q
qRT–PCR for mGPDH, myogenin, and myh3 (L), H&E staining (M), distribution of the fibers CSA (N), qRT–PCR (O), and immunofluorescence staining (P) for utrophin and trichrome staining (Q) in GA muscle from mdx mice 4 weeks after AAV‐mGPDH intramuscular injection.
- R
Exercise capacity of mdx mice 6 weeks after AAV‐mGPDH tail vein injection.
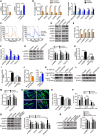
- A–F
Mitochondrial DNA (A), nuclear‐encoded OXPHOS genes (B), respirometry analysis (C), and immunoblots of mGPDH, phospho‐Thr172 AMPK (p‐AMPK), total AMPK (AMPK), phospho‐Ser79‐ACC (p‐ACC), total ACC and PGC1α, and corresponding quantifications represent mGPDH, p‐AMPK, p‐ACC, and PGC1α protein levels (D–F) in C2C12 myocytes transfected with siRNA or plasmid for mGPDH 24 h after differentiation.
- G–I
Immunoblots of p‐AMPK, p‐ACC, and PGC1α and corresponding quantifications represent p‐AMPK, p‐ACC, and PGC1α protein levels (G), mitochondrial DNA (H), and nuclear‐encoded OXPHOS genes combined by NDUFS8, SDHb, Uqcrc1, COX5b, and ATP5a1 (I) in C2C12 myocytes transfected by mGPDH plasmid with the AMPK inhibitor compound C (CC) 24 h after differentiation.
- J, K
NAD+/NADH ratio (J) and immunoprecipitation analysis for PGC1α acetyl‐lysine (Ac‐Lys) level (K) in C2C12 myocytes transfected with siRNA or plasmid for mGPDH 24 h after differentiation.
- L–P
Immunoblot of c‐myc and myogenin (L) and corresponding quantifications represent c‐myc and myogenin protein levels (M), representative images of MyHC immunofluorescence (N), fusion index (O), and the distribution of nuclei per myotube (P) in C2C12 myocytes transfected with mGPDH plasmid with the AMPK inhibitor CC at 24 h (L, M) or 72 h (N–P) after differentiation.
- Q
Immunoblots of p‐AMPK, p‐ACC, PGC1α, and myogenin in C2C12 myocytes transfected with mGPDH plasmid with the CaMKKβ inhibitor STO‐609 at 24 h after differentiation. Quantifications represent p‐AMPK, p‐ACC, PGC1α, and myogenin protein levels.
- R
Immunoblots of p‐AMPK and p‐ACC in C2C12 myocytes transfected with mGPDH plasmid with the Ca2+ chelator BAPTA‐AM at 24 h after differentiation. Quantifications represent p‐AMPK and p‐ACC protein levels.
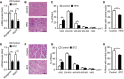
- A–D
Gastrocnemius (GA) muscles were obtained from HFD‐fed mice at day 7 post‐CTX injury. Quantification of myogenin and myh3 by qRT–PCR (A), representative images of H&E staining (B), distribution of the CSA (C), and percentage of myofibers with central nuclei (D).
- E–H
GA muscles were obtained from STZ‐treated mice 4 weeks after STZ injection and at day 7 post‐CTX injury. Quantification of myogenin and myh3 by qRT–PCR (E), representative images of H&E staining (F), distribution of the fibers CSA (G), and percentage of myofibers with central nuclei (H).
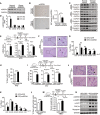
- A–C
Immunoblot (A, C) and IHC (B) of mGPDH and myogenin in GA muscles from obese patients (A, B) and the indicated mice (C).
- D
qRT–PCR of mGPDH in GA muscle of the indicated mice at days 0 and 3 after CTX intramuscular injection.
- E–H
Experimental setup (E, upper panel); qRT–PCR of mGPDH, myogenin, and myh3 (E, bottom panel); H&E staining (arrowhead, necrotic myofibers; asterisks, regenerating fibers) (F); distribution of the fiber CSA (G); and percentage of myofibers with central nuclei (H) in GA muscle from AAV‐mGPDH‐treated HFD‐fed mice at day 7 after CTX intramuscular injection.
- I–M
Experimental setup (I and M, upper panels); qRT–PCR of mGPDH, myogenin, and myh3 (I, bottom panel); H&E staining (arrowhead, necrotic myofibers; asterisks, regenerating fibers) (J); distribution of the fibers CSA (K); percentage of myofibers with central nuclei (L); and muscle weight (M, bottom panel) in GA muscle from AAV‐mGPDH‐treated STZ‐treated mice at days 7 (I–L) and 14 (M) after CTX intramuscular injection.
- N
Immunoblots of mGPDH, p‐AMPK, p‐ACC, PGC1α, and myogenin for the experiment described in (E).

- A–D
Experimental setup (A, upper panel) and qRT–PCR of mGPDH, myogenin, and myh3 gene expressions (A, bottom panel), H&E staining (arrowhead, necrotic myofibers; asterisks, regenerating fibers) (B), distribution of CSA (C), and percentage of fibers with central nuclei (D) in GA muscle from AAV‐mGPDH‐treated ob/ob mice at day 7 post‐CTX.

- A–C
Quantification of the indicated inflammatory cytokines by qRT–PCR in GA muscles of mGPDH−/− mice (A), HFD‐fed and STZ‐treated mice (B), and HFD‐fed and STZ‐treated mice intramuscularly injected with AAV‐mGPDH (C) at day 7 post‐CTX injury.
References
-
- Aragno M, Mastrocola R, Catalano MG, Brignardello E, Danni O, Boccuzzi G (2004) Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes 53: 1082–1088 - PubMed
-
- Benoit B, Meugnier E, Castelli M, Chanon S, Vieille‐Marchiset A, Durand C, Bendridi N, Pesenti S, Monternier PA, Durieux AC et al (2017) Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med 23: 990–996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

