Tolerising cellular therapies: what is their promise for autoimmune disease?
- PMID: 30389690
- PMCID: PMC6390030
- DOI: 10.1136/annrheumdis-2018-214024
Tolerising cellular therapies: what is their promise for autoimmune disease?
Abstract
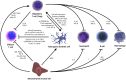
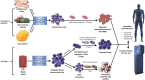
The current management of autoimmunity involves the administration of immunosuppressive drugs coupled to symptomatic and functional interventions such as anti-inflammatory therapies and hormone replacement. Given the chronic nature of autoimmunity, however, the ideal therapeutic strategy would be to reinduce self-tolerance before significant tissue damage has accrued. Defects in, or defective regulation of, key immune cells such as regulatory T cells have been documented in several types of human autoimmunity. Consequently, it has been suggested that the administration of ex vivo generated, tolerogenic immune cell populations could provide a tractable therapeutic strategy. Several potentially tolerogenic cellular therapies have been developed in recent years; concurrent advances in cell manufacturing technologies promise scalable, affordable interventions if safety and efficacy can be demonstrated. These therapies include mesenchymal stromal cells, tolerogenic dendritic cells and regulatory T cells. Each has advantages and disadvantages, particularly in terms of the requirement for a bespoke versus an 'off-the-shelf' treatment but also their suitability in particular clinical scenarios. In this review, we examine the current evidence for these three types of cellular therapy, in the context of a broader discussion around potential development pathway(s) and their likely future role. A brief overview of preclinical data is followed by a comprehensive discussion of human data.
Keywords: Cellular therapies; Crohn’s disease; TR1 cells; autoimmune thyroiditis; graft versus host disease; mesenchymal stromal cells; multiple sclerosis; myasthenia gravis; regulatory T-cells; rheumatoid arthritis; systemic lupus erythematosus; tolerogenic dendritic cells; type 1 diabetes.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann Rheum Dis 2018;77:175–87. 10.1136/annrheumdis-2017-211555 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

