Adipokines Deregulate Cellular Communication via Epigenetic Repression of Gap Junction Loci in Obese Endometrial Cancer
- PMID: 30389702
- PMCID: PMC6705596
- DOI: 10.1158/0008-5472.CAN-18-1615
Adipokines Deregulate Cellular Communication via Epigenetic Repression of Gap Junction Loci in Obese Endometrial Cancer
Abstract
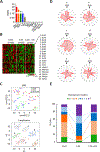
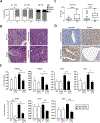
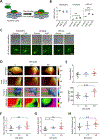
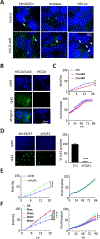
Emerging evidence indicates that adipose stromal cells (ASC) are recruited to enhance cancer development. In this study, we examined the role these adipocyte progenitors play relating to intercellular communication in obesity-associated endometrial cancer. This is particularly relevant given that gap junctions have been implicated in tumor suppression. Examining the effects of ASCs on the transcriptome of endometrial epithelial cells (EEC) in an in vitro coculture system revealed transcriptional repression of GJA1 (encoding the gap junction protein Cx43) and other genes related to intercellular communication. This repression was recapitulated in an obesity mouse model of endometrial cancer. Furthermore, inhibition of plasminogen activator inhibitor 1 (PAI-1), which was the most abundant ASC adipokine, led to reversal of cellular distribution associated with the GJA1 repression profile, suggesting that PAI-1 may mediate actions of ASC on transcriptional regulation in EEC. In an endometrial cancer cohort (n = 141), DNA hypermethylation of GJA1 and related loci TJP2 and PRKCA was observed in primary endometrial endometrioid tumors and was associated with obesity. Pharmacologic reversal of DNA methylation enhanced gap-junction intercellular communication and cell-cell interactions in vitro. Restoring Cx43 expression in endometrial cancer cells reduced cellular migration; conversely, depletion of Cx43 increased cell migration in immortalized normal EEC. Our data suggest that persistent repression by ASC adipokines leads to promoter hypermethylation of GJA1 and related genes in the endometrium, triggering long-term silencing of these loci in endometrial tumors of obese patients. SIGNIFICANCE: Studies reveal that adipose-derived stem cells in endometrial cancer pathogenesis influence epigenetic repression of gap junction loci, which suggests targeting of gap junction activity as a preventive strategy for obesity-associated endometrial cancer.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures


References
-
- Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 2005;105(3):575–80. - PubMed
-
- Kost ER, Valente PT, Lynch BA, Krishnegowda NK, Hertz AM, Hall KL, et al. Clinical and Pathologic Features of Hispanic Endometrial Cancer Patients With Loss of Mismatch Repair Expression. Int J Gynecol Cancer 2016;26(6):1129–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

