Electrical coupling and its channels
- PMID: 30389716
- PMCID: PMC6279368
- DOI: 10.1085/jgp.201812203
Electrical coupling and its channels
Abstract
As the physiology of synapses began to be explored in the 1950s, it became clear that electrical communication between neurons could not always be explained by chemical transmission. Instead, careful studies pointed to a direct intercellular pathway of current flow and to the anatomical structure that was (eventually) called the gap junction. The mechanism of intercellular current flow was simple compared with chemical transmission, but the consequences of electrical signaling in excitable tissues were not. With the recognition that channels were a means of passive ion movement across membranes, the character and behavior of gap junction channels came under scrutiny. It became evident that these gated channels mediated intercellular transfer of small molecules as well as atomic ions, thereby mediating chemical, as well as electrical, signaling. Members of the responsible protein family in vertebrates-connexins-were cloned and their channels studied by many of the increasingly biophysical techniques that were being applied to other channels. As described here, much of the evolution of the field, from electrical coupling to channel structure-function, has appeared in the pages of the Journal of General Physiology.
© 2018 Harris.
Figures
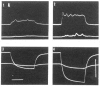
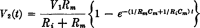
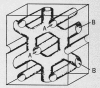
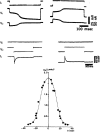



References
-
- Arvanitaki A. 1942. Effects evoked in an axon by the activity of a contiguous one. J. Neurophysiol. 5:89–108. 10.1152/jn.1942.5.2.89 - DOI
-
- Arvanitaki A., and Chalazonitis N.. 1959. [Electrical interactions between the giant soma A and the immediately contiguous somata (pleuro-branchial ganglion of Aplysia ] Bull. Inst. Oceanogr. (1143):1–30.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

