Natural Compounds and Their Analogues as Potent Antidotes against the Most Poisonous Bacterial Toxin
- PMID: 30389764
- PMCID: PMC6275346
- DOI: 10.1128/AEM.01280-18
Natural Compounds and Their Analogues as Potent Antidotes against the Most Poisonous Bacterial Toxin
Abstract
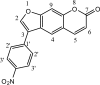
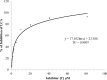
Botulinum neurotoxins (BoNTs), the most poisonous proteins known to humankind, are a family of seven (serotype A to G) immunologically distinct proteins synthesized primarily by different strains of the anaerobic bacterium Clostridium botulinum Being the causative agents of botulism, the toxins block neurotransmitter release by specifically cleaving one of the three soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins, thereby inducing flaccid paralysis. The development of countermeasures and therapeutics against BoNTs is a high-priority research area for public health because of their extreme toxicity and potential for use as biowarfare agents. Extensive research has focused on designing antagonists that block the catalytic activity of BoNTs. In this study, we screened 300 small natural compounds and their analogues extracted from Indian plants for their activity against BoNT serotype A (BoNT/A) as well as its light chain (LCA) using biochemical and cellular assays. One natural compound, a nitrophenyl psoralen (NPP), was identified to be a specific inhibitor of LCA with an in vitro 50% inhibitory concentration (IC50) value of 4.74 ± 0.03 µM. NPP was able to rescue endogenous synaptosome-associated protein 25 (SNAP-25) from cleavage by BoNT/A in human neuroblastoma cells with an IC50 of 12.2 ± 1.7 µM, as well as to prolong the time to the blocking of neutrally elicited twitch tensions in isolated mouse phrenic nerve-hemidiaphragm preparations.IMPORTANCE The long-lasting endopeptidase activity of BoNT is a critical biological activity inside the nerve cell, as it prompts proteolysis of the SNARE proteins, involved in the exocytosis of the neurotransmitter acetylcholine. Thus, the BoNT endopeptidase activity is an appropriate clinical target for designing new small-molecule antidotes against BoNT with the potential to reverse the paralysis syndrome of botulism. In principle, small-molecule inhibitors (SMIs) can gain entry into BoNT-intoxicated cells if they have a suitable octanol-water partition coefficient (log P) value and other favorable characteristics (P. Leeson, Nature 481:455-456, 2012, https://doi.org/10.1038/481455a). Several efforts have been made in the past to develop SMIs, but inhibitors effective under in vitro conditions have not in general been effective in vivo or in cellular models (L. M. Eubanks, M. S. Hixon, W. Jin, S. Hong, et al., Proc Natl Acad Sci U S A 104:2602-2607, 2007, https://doi.org/10.1073/pnas.0611213104). The difference between the in vitro and cellular efficacy presumably results from difficulties experienced by the compounds in crossing the cell membrane, in conjunction with poor bioavailability and high cytotoxicity. The screened nitrophenyl psoralen (NPP) effectively antagonized BoNT/A in both in vitro and ex vivo assays. Importantly, NPP inhibited the BoNT/A light chain but not other general zinc endopeptidases, such as thermolysin, suggesting high selectivity for its target. Small-molecule (nonpeptidic) inhibitors have better oral bioavailability, better stability, and better tissue and cell permeation than antitoxins or peptide inhibitors.
Keywords: IC50; botulinum neurotoxin type A; cell-based SNAP-25 assay; high-throughput screening; phrenic nerve-hemidiaphragm preparation.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

