The Interior Surfaces of Wooden Barrels Are an Additional Microbial Inoculation Source for Lambic Beer Production
- PMID: 30389768
- PMCID: PMC6293109
- DOI: 10.1128/AEM.02226-18
The Interior Surfaces of Wooden Barrels Are an Additional Microbial Inoculation Source for Lambic Beer Production
Abstract
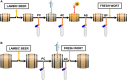
Traditional lambic beer production takes place through wort inoculation with environmental air and fermentation and maturation in wooden barrels. These wooden casks or foeders are possible additional inoculation sources of microorganisms for lambic worts. To date, however, these lambic barrels have been examined only with culture-dependent techniques, thereby missing a portion of the microorganisms present. Moreover, the effects of the cleaning procedures (involving high-pressure water and/or fumigation) and the barrel type on the microbial community structures of the interior surfaces of wooden lambic barrels were unclear. The culture-dependent plating and culture-independent amplicon sequencing of swab samples obtained from the interior surfaces of different wooden casks and foeders used for traditional lambic beer production in Belgium revealed that the microbial compositions of these surfaces differed statistically throughout the barrel-cleaning procedures applied. At the end of the cleaning procedures, amplicon sequencing still detected fermentation- and maturation-related microorganisms, although only a few colonies were still detectable using culture-dependent methods. It is possible that some of the surviving microorganisms were missed due to the presence of many of these cells in a viable but not culturable state and/or engrained deeper in the wood. These surviving microorganisms could act as an additional inoculation source, besides brewery air and brewery equipment, thereby helping to establish a stable microbial community in the wort to diminish batch-to-batch variations in fermentation profiles. Furthermore, the microbial compositions of the interior barrel surfaces differed statistically based on the barrel type, possibly reflecting different characteristics of the lambic barrels in terms of age, wood thickness, and wood porosity.IMPORTANCE Although the coolship step is generally regarded as the main contributor to the spontaneous inoculation by environmental air of fresh worts for lambic beer production, it is known that microorganisms often associate with specific surfaces present in a brewery. However, knowledge about the association of microorganisms with the interior surfaces of wooden lambic barrels is limited. To clarify the role of casks and foeders as additional microbial inoculation sources, it was important to determine the influence of the barrel characteristics and the cleaning procedures on the microbial communities of the interior barrel surfaces. Moreover, this helped to elucidate the complex spontaneous lambic beer fermentation and maturation process. It will allow further optimization of the lambic beer production process, as well as the wooden-barrel-cleaning procedures applied.
Keywords: amplicon sequencing; bacteria; culture-independent analysis; lambic beer; wooden barrels; yeasts.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Leroy F, De Vuyst L. 2004. Functional lactic acid bacteria starter cultures for the food fermentation industry. Trends Food Sci Technol 15:67–78. doi:10.1016/j.tifs.2003.09.004. - DOI
-
- Spitaels F, Wieme AD, Snauwaert I, De Vuyst L, Vandamme P. 2017. Microbial ecology of traditional beer fermentations, p 179–196. In Bokulich N, Bamforth C (ed), Brewing microbiology: current research, omics and microbial ecology. Caister Academic Press, Poole, United Kingdom.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

