The SUMO Conjugation Complex Self-Assembles into Nuclear Bodies Independent of SIZ1 and COP1
- PMID: 30389781
- PMCID: PMC6324245
- DOI: 10.1104/pp.18.00910
The SUMO Conjugation Complex Self-Assembles into Nuclear Bodies Independent of SIZ1 and COP1
Abstract
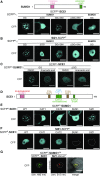
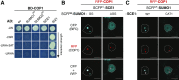
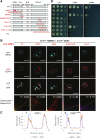
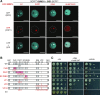
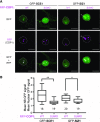
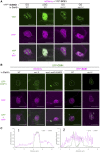
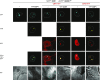
Attachment of the small ubiquitin-like modifier (SUMO) to substrate proteins modulates their turnover, activity, or interaction partners. However, how this SUMO conjugation activity concentrates the proteins involved and the substrates into uncharacterized nuclear bodies (NBs) remains poorly understood. Here, we characterized the requirements for SUMO NB formation and for their subsequent colocalization with the E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), a master regulator of plant growth. COP1 activity results in degradation of transcription factors, which primes the transcriptional response that underlies elongation growth induced by darkness and high ambient temperatures (skoto- and thermomorphogenesis, respectively). SUMO conjugation activity alone was sufficient to target the SUMO machinery into NBs. Colocalization of these bodies with COP1 required, in addition to SUMO conjugation activity, a SUMO acceptor site in COP1 and the SUMO E3 ligase SAP and Miz 1 (SIZ1). We found that SIZ1 docks in the substrate-binding pocket of COP1 via two valine-proline peptide motifs, which represent a known interaction motif of COP1 substrates. The data reveal that SIZ1 physically connects COP1 and SUMO conjugation activity in the same NBs that can also contain the blue-light receptors CRYPTOCHROME 1 and CRYPTOCHROME 2. Our findings thus suggest that sumoylation stimulates COP1 activity within NBs. Moreover, the presence of SIZ1 and SUMO in these NBs explains how both the timing and amplitude of the high-temperature growth response is controlled. The strong colocalization of COP1 and SUMO in these NBs might also explain why many COP1 substrates are sumoylated.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures
References
-
- Bencsath KP, Podgorski MS, Pagala VR, Slaughter CA, Schulman BA (2002) Identification of a multifunctional binding site on Ubc9p required for Smt3p conjugation. J Biol Chem 277: 47938–47945 - PubMed
-
- Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD (2002) Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108: 345–356 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

