Binding of a glaucoma-associated myocilin variant to the αB-crystallin chaperone impedes protein clearance in trabecular meshwork cells
- PMID: 30389787
- PMCID: PMC6311499
- DOI: 10.1074/jbc.RA118.004325
Binding of a glaucoma-associated myocilin variant to the αB-crystallin chaperone impedes protein clearance in trabecular meshwork cells
Abstract
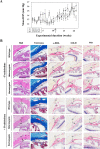
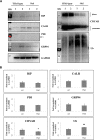
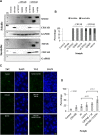
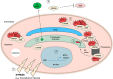
Myocilin (MYOC) was discovered more than 20 years ago and is the gene whose mutations are most commonly observed in individuals with glaucoma. Despite extensive research efforts, the function of WT MYOC has remained elusive, and how mutant MYOC is linked to glaucoma is unclear. Mutant MYOC is believed to be misfolded within the endoplasmic reticulum, and under normal physiological conditions misfolded MYOC should be retro-translocated to the cytoplasm for degradation. To better understand mutant MYOC pathology, we CRISPR-engineered a rat to have a MYOC Y435H substitution that is the equivalent of the pathological human MYOC Y437H mutation. Using this engineered animal model, we discovered that the chaperone αB-crystallin (CRYAB) is a MYOC-binding partner and that co-expression of these two proteins increases protein aggregates. Our results suggest that the misfolded mutant MYOC aggregates with cytoplasmic CRYAB and thereby compromises protein clearance mechanisms in trabecular meshwork cells, and this process represents the primary mode of mutant MYOC pathology. We propose a model by which mutant MYOC causes glaucoma, and we propose that therapeutic treatment of patients having a MYOC mutation may focus on disrupting the MYOC-CRYAB complexes.
Keywords: CRISPR/Cas; aggregation; aging; animal model; cell biology; crystallin; genetic disease; glaucoma; myocilin; secretion.
© 2018 Lynch et al.
Conflict of interest statement
Authors are all employees of Novartis Institutes for BioMedical Research (NIBR) and receive salary. As NIBR is a publicly traded pharmaceutical company, the authors may hold stock
Figures





References
-
- Friedman D. S., Wolfs R. C., O'Colmain B. J., Klein B. E., Taylor H. R., West S., Leske M. C., Mitchell P., Congdon N., Kempen J., and Eye Diseases Prevalence Research Group. (2004) Eye diseases prevalence research group. Prevalence of open-angle glaucoma among adults in the United States. Arch. Ophthalmol. 122, 532–538 10.1001/archopht.122.4.532 - DOI - PMC - PubMed
-
- Meyer A., Béchetoille A., Valtot F., Dupont de Dinechin S., Adam M. F., Belmouden A., Brézin A. P., Gomez L., Bach J. F., and Garchon H. J. (1996) Age-dependent penetrance and mapping of the locus for juvenile and early-onset open-angle glaucoma on chromosome 1q (GLC1A) in a French family. Hum. Genet. 98, 567–571 10.1007/s004390050260 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

