Phosphatidylserine is a marker for axonal debris engulfment but its exposure can be decoupled from degeneration
- PMID: 30389906
- PMCID: PMC6214901
- DOI: 10.1038/s41419-018-1155-z
Phosphatidylserine is a marker for axonal debris engulfment but its exposure can be decoupled from degeneration
Abstract
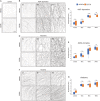
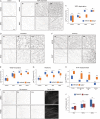
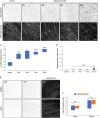
Apoptotic cells expose Phosphatidylserine (PS), that serves as an "eat me" signal for engulfing cells. Previous studies have shown that PS also marks degenerating axonsduring developmental pruning or in response to insults (Wallerian degeneration), but the pathways that control PS exposure on degenerating axons are largely unknown. Here, we used a series of in vitro assays to systematically explore the regulation of PS exposure during axonal degeneration. Our results show that PS exposure is regulated by the upstream activators of axonal pruning and Wallerian degeneration. However, our investigation of signaling further downstream revealed divergence between axon degeneration and PS exposure. Importantly, elevation of the axonal energetic status hindered PS exposure, while inhibition of mitochondrial activity caused PS exposure, without degeneration. Overall, our results suggest that the levels of PS on the outer axonal membrane can be dissociated from the degeneration process and that the axonal energetic status plays a key role in the regulation of PS exposure.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

