Flow cytometric analysis of Xenopus laevis and X. tropicalis blood cells using acridine orange
- PMID: 30390005
- PMCID: PMC6214894
- DOI: 10.1038/s41598-018-34631-0
Flow cytometric analysis of Xenopus laevis and X. tropicalis blood cells using acridine orange
Abstract
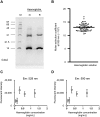
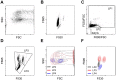
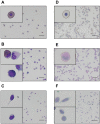
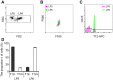
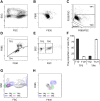
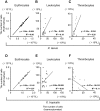
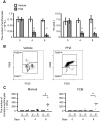
Automated blood cell counters can distinguish cells based on their size and the presence or absence of a nucleus. However, most vertebrates have nucleated blood cells that cannot be counted automatically. We established an alternative automatic method for counting peripheral blood cells by staining cells with the fluorescent dye acridine orange (AO) and analysing cell populations using flow cytometry (FCM). As promising new animal models, we chose Xenopus laevis and three inbred strains of X. tropicalis. We compared the haematological phenotypes, including blood cell types, cell sizes, cellular structure, and erythrocyte lifespans/turnover rate among X. laevis and the three inbred strains of X. tropicalis. Each cell type from X. laevis was sorted according to six parameters: forward- and side-scattered light emission, AO red and green fluorescence intensity, and cellular red and green fluorescence. Remarkably, the erythrocyte count was the highest in the Golden line, suggesting that genetic factors were associated with the blood cells. Furthermore, immature erythrocytes in anaemic X. laevis could be separated from normal blood cells based on red fluorescence intensity. These results show that FCM with AO staining allows for an accurate analysis of peripheral blood cells from various species.
Conflict of interest statement
Y. N. is an employee of NIHON KOHDEN CORPORATION.
Figures
Similar articles
-
A new method for fast blood cell counting and partial differentiation by flow cytometry.Blut. 1984 Aug;49(2):83-90. doi: 10.1007/BF00363698. Blut. 1984. PMID: 6205708
-
A new method for fish leucocyte counting and partial differentiation by flow cytometry.Fish Shellfish Immunol. 2002 Nov;13(5):379-90. doi: 10.1006/fsim.2002.0413. Fish Shellfish Immunol. 2002. PMID: 12458744
-
Evaluating Blood Cell Populations in Xenopus Using Flow Cytometry and Differential Counts by Cytospin.Methods Mol Biol. 2018;1865:265-273. doi: 10.1007/978-1-4939-8784-9_19. Methods Mol Biol. 2018. PMID: 30151773
-
Overview of flow cytometry and fluorescent probes for cytometry.Methods Mol Biol. 1994;34:219-24. doi: 10.1385/0-89603285-x:219. Methods Mol Biol. 1994. PMID: 7536077 Review. No abstract available.
-
Fluorescent cell barcoding for multiplex flow cytometry.Curr Protoc Cytom. 2011 Jan;Chapter 6:6.31.1-6.31.15. doi: 10.1002/0471142956.cy0631s55. Curr Protoc Cytom. 2011. PMID: 21207359 Free PMC article. Review.
Cited by
-
Tissue disaggregation and isolation of specific cell types from transgenic Xenopus appendages for transcriptional analysis by FACS.Dev Dyn. 2021 Sep;250(9):1381-1392. doi: 10.1002/dvdy.268. Epub 2020 Nov 12. Dev Dyn. 2021. PMID: 33137227 Free PMC article.
-
Mid-Tibiofibular Amputation as a Method of Terminal Blood Collection in Xenopus Laevis.J Am Assoc Lab Anim Sci. 2021 Sep 1;60(5):582-586. doi: 10.30802/AALAS-JAALAS-21-000005. Epub 2021 Jul 15. J Am Assoc Lab Anim Sci. 2021. PMID: 34266520 Free PMC article.
-
Isolation and evaluation of erythroid progenitors in the livers of larval, froglet, and adult Xenopus tropicalis.Biol Open. 2023 Aug 15;12(8):bio059862. doi: 10.1242/bio.059862. Epub 2023 Jul 27. Biol Open. 2023. PMID: 37421150 Free PMC article.
-
Xenopus tropicalis: Joining the Armada in the Fight Against Blood Cancer.Front Physiol. 2019 Feb 1;10:48. doi: 10.3389/fphys.2019.00048. eCollection 2019. Front Physiol. 2019. PMID: 30774603 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

