Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence
- PMID: 30390064
- PMCID: PMC6333805
- DOI: 10.1038/s41386-018-0257-8
Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence
Abstract
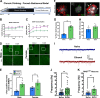
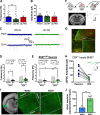
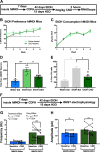
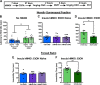
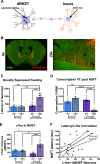
Negative affect is a core symptom domain associated with an array of neurological and psychiatric disorders and is only partially targeted by current therapies, highlighting the need for better, more targeted treatment options. This study focuses on negative affective symptoms associated with prolonged alcohol abstinence, one of the leading causes of relapse. Using a mouse model of chronic alcohol consumption followed by forced abstinence (CDFA), prolonged alcohol abstinence increased c-fos expression and spontaneous glutamatergic neurotransmission in the dorsal bed nucleus of the stria terminalis (dBNST), a region heavily implicated in negative affect in both humans and rodents. Further, pharmacologically enhancing endogenous cannabinoids (eCB) with JZL184 prevents abstinence-induced increases in dBNST neuronal activity, underscoring the therapeutic potential of drugs targeting the brain's eCB system. Next, we used a channelrhodopsin-assisted mapping strategy to identify excitatory inputs to the dBNST that could contribute to CDFA-induced negative affect. We identified the insular cortex (insula), a region involved in regulating interoception, as a dense, functional, eCB-sensitive input to the dBNST. Using a chemogenetic strategy to locally mimic eCB signaling, we demonstrate that the insula strongly influences the CDFA behavioral phenotype and dBNST neuronal activity. Lastly, we used an anterograde strategy for transynaptic targeting of Cre expression in combination with a Gq-DREADD to selectively recruit dBNST neurons receiving insula projections. Chemogenetic recruitment of these neurons mimicked behavioral and c-fos responses observed in CDFA. Collectively, this study supports a role for the insula-BNST neural circuit in negative affective disturbances and highlights the therapeutic potential of the eCB system for treating negative affective disorders.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

