Epigenetic fates of gene silencing established by heterochromatin spreading in cell identity and genome stability
- PMID: 30390097
- PMCID: PMC6452905
- DOI: 10.1007/s00294-018-0901-1
Epigenetic fates of gene silencing established by heterochromatin spreading in cell identity and genome stability
Abstract
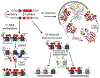
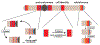
Heterochromatin spreading, the propagation of repressive chromatin along the chromosome, is a reaction critical to genome stability and defense, as well as maintenance of unique cell fates. Here, we discuss the intrinsic properties of the spreading reaction and circumstances under which its products, formed distal to DNA-encoded nucleation sites, can be epigenetically maintained. Finally, we speculate that the epigenetic properties of heterochromatin evolved together with the need to stabilize cellular identity.
Keywords: Cellular identity; Chromatin structure; DNA methylation; Epigenetic inheritance; Heterochromatin spreading; Histone 3 lysine 9 methylation; Histone turnover.
Conflict of interest statement
Compliance with ethical standards
The authors declare that they have no conflicts of interest. No human subjects or animals were used in this study.
Figures

Similar articles
-
Local chromatin context regulates the genetic requirements of the heterochromatin spreading reaction.PLoS Genet. 2022 May 18;18(5):e1010201. doi: 10.1371/journal.pgen.1010201. eCollection 2022 May. PLoS Genet. 2022. PMID: 35584134 Free PMC article.
-
Ten principles of heterochromatin formation and function.Nat Rev Mol Cell Biol. 2018 Apr;19(4):229-244. doi: 10.1038/nrm.2017.119. Epub 2017 Dec 13. Nat Rev Mol Cell Biol. 2018. PMID: 29235574 Free PMC article. Review.
-
Uncoupling of genomic and epigenetic signals in the maintenance and inheritance of heterochromatin domains in fission yeast.Genetics. 2012 Feb;190(2):549-57. doi: 10.1534/genetics.111.137083. Epub 2011 Dec 5. Genetics. 2012. PMID: 22143918 Free PMC article.
-
Noncoding RNA-nucleated heterochromatin spreading is intrinsically labile and requires accessory elements for epigenetic stability.Elife. 2018 Jul 18;7:e32948. doi: 10.7554/eLife.32948. Elife. 2018. PMID: 30020075 Free PMC article.
-
Role of H3K9me3 heterochromatin in cell identity establishment and maintenance.Curr Opin Genet Dev. 2019 Apr;55:1-10. doi: 10.1016/j.gde.2019.04.013. Epub 2019 May 16. Curr Opin Genet Dev. 2019. PMID: 31103921 Free PMC article. Review.
Cited by
-
Genome protection: histone H4 and beyond.Curr Genet. 2020 Oct;66(5):945-950. doi: 10.1007/s00294-020-01088-6. Epub 2020 Jun 17. Curr Genet. 2020. PMID: 32556547 Free PMC article. Review.
-
Transgenerational effect of drug-mediated inhibition of LSD1 on eye pigment expression in Drosophila.BMC Ecol. 2020 Nov 23;20(1):62. doi: 10.1186/s12898-020-00330-6. BMC Ecol. 2020. PMID: 33228645 Free PMC article.
-
Monitoring of switches in heterochromatin-induced silencing shows incomplete establishment and developmental instabilities.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):20043-20053. doi: 10.1073/pnas.1909724116. Epub 2019 Sep 16. Proc Natl Acad Sci U S A. 2019. PMID: 31527269 Free PMC article.
-
Heterochromatin organization and phase separation.Nucleus. 2023 Dec;14(1):2159142. doi: 10.1080/19491034.2022.2159142. Nucleus. 2023. PMID: 36710442 Free PMC article. Review.
References
-
- Reinberg D and Vales LD, Chromatin domains rich in inheritance. Science, 2018. 361(6397): p. 33–34. - PubMed

