CuI -Mediated Bromoalkynylation and Hydroalkynylation Reactions of Unsymmetrical Benzynes: Complementary Modes of Addition
- PMID: 30390400
- PMCID: PMC6386157
- DOI: 10.1002/anie.201811783
CuI -Mediated Bromoalkynylation and Hydroalkynylation Reactions of Unsymmetrical Benzynes: Complementary Modes of Addition
Abstract
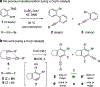
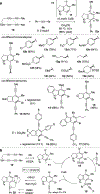
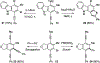
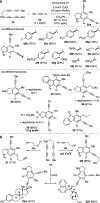
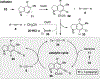
Benzynes formed by heating a suitable triyne (or tetrayne) substrate are shown to react with in situ generated alkynyl copper species. The latter are compatible with the polyyne substrates and two types of chemistries have been achieved: (i) 1-bromo-1-alkynes efficiently undergo net bromoalkynylation of the (unsymmetrical) benzynes and (ii) in situ generated alkynylcopper species give rise to hydroalkynylation products. The regiochemical preferences of these two modes of reaction are complementary to one another with respect to the position of alkynyl substituent in the final products.
Keywords: alkynylations; aryne trapping; copper; homogeneous catalysis.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Hexadehydro-Diels-Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes.Chem Rev. 2021 Feb 24;121(4):2413-2444. doi: 10.1021/acs.chemrev.0c00825. Epub 2021 Jan 25. Chem Rev. 2021. PMID: 33492939 Free PMC article. Review.
-
The Phenol-Ene Reaction: Biaryl Synthesis via Trapping Reactions between HDDA-Generated Benzynes and Phenolics.Org Lett. 2016 Nov 4;18(21):5596-5599. doi: 10.1021/acs.orglett.6b02830. Epub 2016 Oct 21. Org Lett. 2016. PMID: 27767316 Free PMC article.
-
The domino hexadehydro-Diels-Alder reaction transforms polyynes to benzynes to naphthynes to anthracynes to tetracynes (and beyond?).Nat Chem. 2018 Aug;10(8):838-844. doi: 10.1038/s41557-018-0075-y. Epub 2018 Jul 20. Nat Chem. 2018. PMID: 30030536 Free PMC article.
-
Palladium-catalyzed three-component coupling of arynes with allylic acetates or halides and terminal alkynes promoted by cuprous iodide.Chem Commun (Camb). 2008 May 14;(18):2158-60. doi: 10.1039/b800118a. Epub 2008 Mar 3. Chem Commun (Camb). 2008. PMID: 18438501
-
[Development of Efficient Methods for Benzyne Generation].Yakugaku Zasshi. 2018;138(1):27-35. doi: 10.1248/yakushi.17-00157. Yakugaku Zasshi. 2018. PMID: 29311462 Review. Japanese.
Cited by
-
Cu(I)-Catalyzed 1,2-Alkynyl-propargylation and -benzylation of Benzyne Derivatives.Org Lett. 2021 Jul 16;23(14):5448-5451. doi: 10.1021/acs.orglett.1c01788. Epub 2021 Jun 28. Org Lett. 2021. PMID: 34180676 Free PMC article.
-
Metal-Catalyzed Haloalkynylation Reactions.Chemistry. 2022 Jan 19;28(4):e202103046. doi: 10.1002/chem.202103046. Epub 2021 Nov 18. Chemistry. 2022. PMID: 34644433 Free PMC article. Review.
-
Hexadehydro-Diels-Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes.Chem Rev. 2021 Feb 24;121(4):2413-2444. doi: 10.1021/acs.chemrev.0c00825. Epub 2021 Jan 25. Chem Rev. 2021. PMID: 33492939 Free PMC article. Review.
-
1,3-Butadiynamides the Ethynylogous Ynamides: Synthesis, Properties and Applications in Heterocyclic Chemistry.Molecules. 2023 Jun 5;28(11):4564. doi: 10.3390/molecules28114564. Molecules. 2023. PMID: 37299038 Free PMC article. Review.
References
-
- Morishita T, Yoshida H, Ohshita J, Chem. Commun 2010, 46, 640. - PubMed
-
- Himeshima Y, Sonoda T, Kobayashi H, Chem. Lett 1983, 12, 1211.
-
- Various Cu(I) salts have been reported to oligomerize Kobayashi benzynes: Mizukoshi Y, Mikami K, Uchiyama M, J. Am. Chem. Soc 2015, 137, 74. - PubMed
-
- a)Miyawaki K, Suzuki R, Kawano T, Ueda I, Tetrahedron Lett 1997, 38, 3943;
- b)Bradley AZ, Johnson RP, J. Am. Chem. Soc 1997, 119, 9917;
- c)Tsui JA, Sterenberg BT, Organometallics 2009, 28, 4906;
- d)Hoye TR, Baire B, Niu D, Willoughby PH, Woods BP, Nature 2012, 490, 208; - PMC - PubMed
- e)Karmakar R, Mamidipalli P, Yun SY, Lee D, Org. Lett 2013, 15, 1938; - PubMed
- f) Diamond OJ, Marder TB, Org. Chem. Front 2017, 4, 891.
-
- a)Xie CS, Liu LF, Zhang YH, Xu PX, Org. Lett 2008, 10, 2393; - PubMed
- b)Jeganmohan M, Bhuvaneswari S, Cheng CH, Angew. Chem. Int. Ed 2009, 48, 391; - PubMed
- c)Yoshida H, Morishita T, Nakata H, Ohshita J, Org. Lett 2009, 11, 373; - PubMed
- d)Akubathini SK, Biehl E, Tetrahedron Lett 2009, 50, 1809;
- e)Kerisit N, Toupet L, Larini P, Perrin L, Guillemin J-C, Trolez Y, Chem. Eur. J 2015, 21, 6042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

