Balanced Activity between Kv3 and Nav Channels Determines Fast-Spiking in Mammalian Central Neurons
- PMID: 30390433
- PMCID: PMC6218699
- DOI: 10.1016/j.isci.2018.10.014
Balanced Activity between Kv3 and Nav Channels Determines Fast-Spiking in Mammalian Central Neurons
Abstract
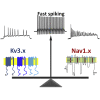
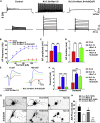
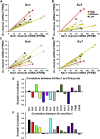
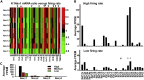
Fast-spiking (FS) neurons can fire action potentials (APs) up to 1,000 Hz and play key roles in vital functions such as sound location, motor coordination, and cognition. Here we report that the concerted actions of Kv3 voltage-gated K+ (Kv) and Na+ (Nav) channels are sufficient and necessary for inducing and maintaining FS. Voltage-clamp analysis revealed a robust correlation between the Kv3/Nav current ratio and FS. Expressing Kv3 channels alone could convert ∼30%-60% slow-spiking (SS) neurons to FS in culture. In contrast, co-expression of either Nav1.2 or Nav1.6 together with Kv3.1 or Kv3.3, but not alone or with Kv1.2, converted SS to FS with 100% efficiency. Furthermore, RNA-sequencing-based genome-wide analysis revealed that the Kv3/Nav ratio and Kv3 expression levels strongly correlated with the maximal AP frequencies. Therefore, FS is established by the properly balanced activities of Kv3 and Nav channels and could be further fine-tuned by channel biophysical features and localization patterns.
Keywords: Biophysics; Molecular Neuroscience; Neuroscience.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Specific functions of synaptically localized potassium channels in synaptic transmission at the neocortical GABAergic fast-spiking cell synapse.J Neurosci. 2005 May 25;25(21):5230-5. doi: 10.1523/JNEUROSCI.0722-05.2005. J Neurosci. 2005. PMID: 15917463 Free PMC article.
-
Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons.J Neurophysiol. 1999 Nov;82(5):2476-89. doi: 10.1152/jn.1999.82.5.2476. J Neurophysiol. 1999. PMID: 10561420
-
Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons.J Neurosci. 2003 Mar 15;23(6):2058-68. doi: 10.1523/JNEUROSCI.23-06-02058.2003. J Neurosci. 2003. PMID: 12657664 Free PMC article.
-
Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing.Trends Neurosci. 2001 Sep;24(9):517-26. doi: 10.1016/s0166-2236(00)01892-0. Trends Neurosci. 2001. PMID: 11506885 Review.
-
When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons.J Neurobiol. 1998 Oct;37(1):69-79. doi: 10.1002/(sici)1097-4695(199810)37:1<69::aid-neu6>3.0.co;2-6. J Neurobiol. 1998. PMID: 9777733 Review.
Cited by
-
Discovering functional interactions among schizophrenia-risk genes by combining behavioral genetics with cell biology.Neurosci Biobehav Rev. 2024 Dec;167:105897. doi: 10.1016/j.neubiorev.2024.105897. Epub 2024 Sep 14. Neurosci Biobehav Rev. 2024. PMID: 39278606 Free PMC article. Review.
-
Progressive myoclonus epilepsy KCNC1 variant causes a developmental dendritopathy.Epilepsia. 2021 May;62(5):1256-1267. doi: 10.1111/epi.16867. Epub 2021 Mar 18. Epilepsia. 2021. PMID: 33735526 Free PMC article.
-
In Search of Transcriptomic Correlates of Neuronal Firing-Rate Adaptation across Subtypes, Regions and Species: A Patch-seq Analysis.bioRxiv [Preprint]. 2024 Dec 10:2024.12.05.627057. doi: 10.1101/2024.12.05.627057. bioRxiv. 2024. PMID: 39713292 Free PMC article. Preprint.
-
Biophysical modelling of intrinsic cardiac nervous system neuronal electrophysiology based on single-cell transcriptomics.J Physiol. 2025 Mar;603(7):2119-2138. doi: 10.1113/JP287595. Epub 2025 Mar 12. J Physiol. 2025. PMID: 40077928 Free PMC article.
-
Interneuron diversity in the human dorsal striatum.Nat Commun. 2024 Jul 22;15(1):6164. doi: 10.1038/s41467-024-50414-w. Nat Commun. 2024. PMID: 39039043 Free PMC article.
References
-
- Bean B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8:451–465. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

