Transcriptome analysis of Xenopus orofacial tissues deficient in retinoic acid receptor function
- PMID: 30390632
- PMCID: PMC6215681
- DOI: 10.1186/s12864-018-5186-8
Transcriptome analysis of Xenopus orofacial tissues deficient in retinoic acid receptor function
Abstract
Background: Development of the face and mouth is orchestrated by a large number of transcription factors, signaling pathways and epigenetic regulators. While we know many of these regulators, our understanding of how they interact with each other and implement changes in gene expression during orofacial development is still in its infancy. Therefore, this study focuses on uncovering potential cooperation between transcriptional regulators and one important signaling pathway, retinoic acid, during development of the midface.
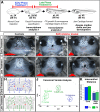
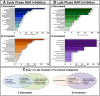
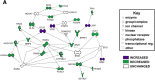
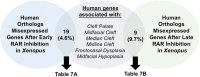
Results: Transcriptome analyses was performed on facial tissues deficient for retinoic acid receptor function at two time points in development; early (35 hpf) just after the neural crest migrates and facial tissues are specified and later (60 hpf) when the mouth has formed and facial structures begin to differentiate. Functional and network analyses revealed that retinoic acid signaling could cooperate with novel epigenetic factors and calcium-NFAT signaling during early orofacial development. At the later stage, retinoic acid may work with WNT and BMP and regulate homeobox containing transcription factors. Finally, there is an overlap in genes dysregulated in Xenopus embryos with median clefts with human genes associated with similar orofacial defects.
Conclusions: This study uncovers novel signaling pathways required for orofacial development as well as pathways that could interact with retinoic acid signaling during the formation of the face. We show that frog faces are an important tool for studying orofacial development and birth defects.
Keywords: Cleft palate; Orofacial development; Retinoic acid; Transcriptomics; Xenopus laevis.
Conflict of interest statement
Ethics approval and consent to participate
All procedures using animals were approved by the VCU Institutional Animal Care and Use Committee (IACUC protocol number 5 AD20261).
Consent for publication
Not applicable to this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

