DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder
- PMID: 30390684
- PMCID: PMC6215613
- DOI: 10.1186/s13148-018-0569-x
DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder
Abstract
Background: We have previously evaluated the efficacy of the CRF1 receptor antagonist GSK561679 in female PTSD patients. While GSK561679 was not superior to placebo overall, it was associated with a significantly stronger symptom reduction in a subset of patients with probable CRF system hyperactivity, i.e., patients with child abuse and CRHR1 SNP rs110402 GG carriers. Here, we test whether blood-based DNA methylation levels within CRHR1 and other PTSD-relevant genes would be associated with treatment outcome, either overall or in the high CRF activity subgroup.
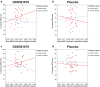
Results: Therefore, we measured CRHR1 genotypes as well as baseline and post-treatment DNA methylation from the peripheral blood in the same cohort of PTSD-diagnosed women treated with GSK561679 (N = 43) or placebo (N = 45). In the same patients, we assessed DNA methylation at the PTSD-relevant genes NR3C1 and FKBP5, shown to predict or associate with PTSD treatment outcome after psychotherapy. We observed significant differences in CRHR1 methylation after GSK561679 treatment in the subgroup of patients with high CRF activity. Furthermore, NR3C1 baseline methylation significantly interacted with child abuse to predict PTSD symptom change following GSK561679 treatment.
Conclusions: Our results support a possible role of CRHR1 methylation levels as an epigenetic marker to track response to CRF1 antagonist treatment in biologically relevant subgroups. Moreover, pre-treatment NR3C1 methylation levels may serve as a potential marker to predict PTSD treatment outcome, independent of the type of therapy. However, to establish clinical relevance of these markers, our findings require replication and validation in larger studies.
Trial registration: NCT01018992 . Registered 6 November 2009.
Keywords: CRF1 receptor antagonist; CRHR1; DNA methylation; Epigenetics; FKBP5; NR3C1; PTSD.
Conflict of interest statement
Ethics approval and consent to participate
The study is being conducted in accord with the latest version of the Declaration of Helsinki. Each site’s Institutional Review Board (IRB) approved the study design, procedures, and recruitment strategies, with Emory University serving as the lead site (Emory University IRB, IRB number 00022717; Mount Sinai School of Medicine IRB, IRB number 04-0900 0001 03; Baylor College of Medicine IRB, IRB number H-30433 and the Michael E DeBakey Veterans Affairs Medical Center Research and Development Program, ID number 12G19, HBP; University of California San Francisco IRB, and the San Francisco Veterans Affairs Research and Development Committee, IRB number 12-09929). The study is registered at
Consent for publication
Not applicable
Competing interests
Dr. Mayberg reports grants from NIMH, grants, and other from GSK, during the conduct of the study; personal fees from Abbott Labs (previously St Jude Medical Inc), outside the submitted work.
Dr. Dunlop reports grants from the National Institute of Mental Health during the conduct of the study; grants from Takeda, grants from Janssen, grants from Acadia, grants from Axsome, outside the submitted work.
Dr. Mathew has served as a consultant to Allergan, Alkermes, and Fortress Biotech. He has received research support from NeuroRx.
Dr. Iosifescu reports grants from the National Institute of Mental Health, during the conduct of the study; personal fees from Axsome, personal fees from Alkermes, grants from Brainsway, grants from LiteCure, personal fees from Lundbeck, grants from Neosync, personal fees from Otsuka, personal fees from Sunovion, outside the submitted work.
Dr. Nemeroff has received research support from the National Institutes of Health (NIH) and Stanley Medical Research Institute. For the last 3 years, he was a consultant for Xhale, Takeda, Taisho Pharmaceutical Inc., Prismic Pharmaceuticals, Bracket (Clintara), Fortress Biotech, Sunovion Pharmaceuticals Inc., Sumitomo Dainippon Pharma, Janssen Research & Development LLC, Magstim, Inc., Navitor Pharmaceuticals, Inc., TC MSO, Inc., Intra-Cellular Therapies, Inc. He is a Stockholder of Xhale, Celgene, Seattle Genetics, Abbvie, OPKO Health, Antares, BI Gen Holdings, Inc., and Corcept Therapeutics Pharmaceuticals Company. Dr. Nemeroff is a member of the scientific advisory boards of the American Foundation for Suicide Prevention (AFSP), Brain and Behavior Research Foundation (BBRF) (formerly named National Alliance for Research on Schizophrenia and Depression [NARSAD]), Xhale, Anxiety Disorder Association of America (ADAA), Skyland Trail, Bracket (Clintara), Laureate Institute for Brain Research, Inc. and on the board of directors of AFSP, Gratitude America and ADAA. Dr. Nemeroff has income sources or equity of 10.000 USD or more from American Psychiatric Publishing, Xhale, Bracket (Clintara), CME Outfitters, Takeda, Intra-Cellular Therapies, Inc., and Magstim. Dr. Nemeroff holds the following patents: Method and devices for transdermal delivery of lithium (US 6,375,990B1) and Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitter by ex vivo assay (US 7,148,027B2).
Dr. Binder, Dr. Rothbaum, Dr. Neylan, Dr. Pape, Dr. Carrillo-Roa, Dr. Czamara and Dr. Zannas have nothing to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Krystal John H., Davis Lori L., Neylan Thomas C., A. Raskind Murray, Schnurr Paula P., Stein Murray B., Vessicchio Jennifer, Shiner Brian, Gleason Theresa D., Huang Grant D. It Is Time to Address the Crisis in the Pharmacotherapy of Posttraumatic Stress Disorder: A Consensus Statement of the PTSD Psychopharmacology Working Group. Biological Psychiatry. 2017;82(7):e51–e59. doi: 10.1016/j.biopsych.2017.03.007. - DOI - PubMed
-
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

