Type 2 diabetes increases oocyte mtDNA mutations which are eliminated in the offspring by bottleneck effect
- PMID: 30390692
- PMCID: PMC6215660
- DOI: 10.1186/s12958-018-0423-1
Type 2 diabetes increases oocyte mtDNA mutations which are eliminated in the offspring by bottleneck effect
Abstract
Background: Diabetes induces many complications including reduced fertility and low oocyte quality, but whether it causes increased mtDNA mutations is unknown.
Methods: We generated a T2D mouse model by using high-fat-diet (HFD) and Streptozotocin (STZ) injection. We examined mtDNA mutations in oocytes of diabetic mice by high-throughput sequencing techniques.
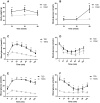
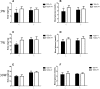
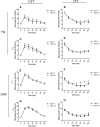
Results: T2D mice showed glucose intolerance, insulin resistance, low fecundity compared to the control group. T2D oocytes showed increased mtDNA mutation sites and mutation numbers compared to the control counterparts. mtDNA mutation examination in F1 mice showed that the mitochondrial bottleneck could eliminate mtDNA mutations.
Conclusions: T2D mice have increased mtDNA mutation sites and mtDNA mutation numbers in oocytes compared to the counterparts, while these adverse effects can be eliminated by the bottleneck effect in their offspring. This is the first study using a small number of oocytes to examine mtDNA mutations in diabetic mothers and offspring.
Keywords: Bottleneck; Diabetes; Oocyte; mtDNA mutation.
Conflict of interest statement
Ethics approval and consent to participate
All procedures described were reviewed and approved by the ethical committee of the Institute of Zoology, Chinese Academy of Sciences. All animal care and use procedures were in accordance with guidelines of the Institutional Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
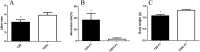
Similar articles
-
Very low-level heteroplasmy mtDNA variations are inherited in humans.J Genet Genomics. 2013 Dec 20;40(12):607-15. doi: 10.1016/j.jgg.2013.10.003. Epub 2013 Dec 8. J Genet Genomics. 2013. PMID: 24377867 Free PMC article.
-
Oocyte mitochondrial deletions and heteroplasmy in a bovine model of ageing and ovarian stimulation.Mol Hum Reprod. 2016 Apr;22(4):261-71. doi: 10.1093/molehr/gaw003. Epub 2016 Jan 20. Mol Hum Reprod. 2016. PMID: 26792869
-
Mitochondrial DNA and the mammalian oocyte.Curr Top Dev Biol. 2007;77:87-111. doi: 10.1016/S0070-2153(06)77004-1. Curr Top Dev Biol. 2007. PMID: 17222701 Review.
-
Effects of adverse fertility-related factors on mitochondrial DNA in the oocyte: a comprehensive review.Reprod Biol Endocrinol. 2023 Mar 17;21(1):27. doi: 10.1186/s12958-023-01078-6. Reprod Biol Endocrinol. 2023. PMID: 36932444 Free PMC article. Review.
-
Deleterious mtDNA mutations are common in mature oocytes.Biol Reprod. 2020 Mar 13;102(3):607-619. doi: 10.1093/biolre/ioz202. Biol Reprod. 2020. PMID: 31621839 Free PMC article.
Cited by
-
Aerobic exercise improves cognitive impairment in mice with type 2 diabetes by regulating the MALAT1/miR-382-3p/BDNF signaling pathway in serum-exosomes.Mol Med. 2023 Sep 22;29(1):130. doi: 10.1186/s10020-023-00727-1. Mol Med. 2023. PMID: 37740187 Free PMC article.
-
High-Fat Diet and Female Fertility across Lifespan: A Comparative Lesson from Mammal Models.Nutrients. 2022 Oct 17;14(20):4341. doi: 10.3390/nu14204341. Nutrients. 2022. PMID: 36297035 Free PMC article. Review.
-
Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality.Cancers (Basel). 2019 Sep 19;11(9):1402. doi: 10.3390/cancers11091402. Cancers (Basel). 2019. PMID: 31546918 Free PMC article. Review.
-
Effect of in vitro growth on mouse oocyte competency, mitochondria and transcriptome.Reproduction. 2021 Sep 9;162(4):307-318. doi: 10.1530/REP-21-0209. Reproduction. 2021. PMID: 34397394 Free PMC article.
-
Ablation of Sam50 is associated with fragmentation and alterations in metabolism in murine and human myotubes.bioRxiv [Preprint]. 2023 Oct 19:2023.05.20.541602. doi: 10.1101/2023.05.20.541602. bioRxiv. 2023. PMID: 37292887 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

