Orphan nuclear receptor TR3/Nur77 differentially regulates the expression of integrins in angiogenesis
- PMID: 30391133
- PMCID: PMC6294663
- DOI: 10.1016/j.mvr.2018.10.011
Orphan nuclear receptor TR3/Nur77 differentially regulates the expression of integrins in angiogenesis
Abstract
Pathological angiogenesis is a hallmark of many diseases. Previously, we reported that orphan nuclear receptor TR3/Nur77 (human homolog, Nur77, mouse homolog) is a critical mediator of angiogenesis to regulate tumor growth and skin wound healing via down-regulating the expression of the junctional proteins and integrin β4. However, the molecular mechanism, by which TR3/Nur77 regulated angiogenesis, was still not completely understood. In this report by analyzing the integrin expression profile in endothelial cells, we found that the TR3/Nur77 expression highly increased the expression of integrins α1 and β5, decreased the expression of integrins α2 and β3, but had some or no effect on the expression of integrins αv, α3, α4, α5, α6, β1 and β7. In the angiogenic responses mediated by TR3/Nur77, integrin α1 regulated endothelial cell proliferation and adhesion, but not migration. Integrin β5 shRNA inhibited cell migration, but increased proliferation and adhesion. Integrin α2 regulated all of the endothelial cell proliferation, migration and adhesion. However, integrin β3 did not play any role in endothelial cell proliferation, migration and adhesion. TR3/Nur77 regulated the transcription of integrins α1, α2, β3 and β5, via various amino acid fragments within its transactivation domain and DNA binding domain. Furthermore, TR3/Nur77 regulated the integrin α1 promoter activity by directly interacting with a novel DNA element within the integrin α1 promoter. These studies furthered our understanding of the molecular mechanism by which TR3/Nur77 regulated angiogenesis, and supported our previous finding that TR3/Nur77 was an excellent therapeutic target for pathological angiogenesis. Therefore, targeting TR3/Nur77 inhibits several signaling pathways that are activated by various angiogenic factors.
Keywords: Angiogenesis; Integrin; Migration; Proliferation; Promoter; TR3/Nur77.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
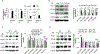

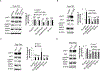




Similar articles
-
DLL4 and Jagged1 are angiogenic targets of orphan nuclear receptor TR3/Nur77.Microvasc Res. 2019 Jul;124:67-75. doi: 10.1016/j.mvr.2019.03.006. Epub 2019 Mar 28. Microvasc Res. 2019. PMID: 30930165 Free PMC article.
-
Orphan nuclear receptor TR3/Nur77 improves wound healing by upregulating the expression of integrin β4.FASEB J. 2015 Jan;29(1):131-40. doi: 10.1096/fj.14-257550. Epub 2014 Oct 17. FASEB J. 2015. PMID: 25326539 Free PMC article.
-
Orphan nuclear receptor TR3/Nur77 biologics inhibit tumor growth by targeting angiogenesis and tumor cells.Microvasc Res. 2020 Mar;128:103934. doi: 10.1016/j.mvr.2019.103934. Epub 2019 Oct 23. Microvasc Res. 2020. PMID: 31654655
-
Endothelial cell integrins and COX-2: mediators and therapeutic targets of tumor angiogenesis.Biochim Biophys Acta. 2004 Mar 4;1654(1):51-67. doi: 10.1016/j.bbcan.2003.09.003. Biochim Biophys Acta. 2004. PMID: 14984767 Review.
-
Dual roles of orphan nuclear receptor TR3/Nur77/NGFI-B in mediating cell survival and apoptosis.Int Rev Cell Mol Biol. 2014;313:219-58. doi: 10.1016/B978-0-12-800177-6.00007-4. Int Rev Cell Mol Biol. 2014. PMID: 25376494 Review.
Cited by
-
TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12306-12314. doi: 10.1073/pnas.1915681117. Epub 2020 May 21. Proc Natl Acad Sci U S A. 2020. PMID: 32439709 Free PMC article.
-
Targeting NR4A Nuclear Receptors to Control Stromal Cell Inflammation, Metabolism, Angiogenesis, and Tumorigenesis.Front Cell Dev Biol. 2021 Feb 9;9:589770. doi: 10.3389/fcell.2021.589770. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33634114 Free PMC article. Review.
-
NR4A1 Acts as a Nutrient Sensor That Inhibits the Effects of Aging.Nutrients. 2025 Aug 21;17(16):2709. doi: 10.3390/nu17162709. Nutrients. 2025. PMID: 40871737 Free PMC article. Review.
-
PROTAC-mediated NR4A1 degradation as a novel strategy for cancer immunotherapy.J Exp Med. 2024 Mar 4;221(3):e20231519. doi: 10.1084/jem.20231519. Epub 2024 Feb 9. J Exp Med. 2024. PMID: 38334978 Free PMC article.
-
Identification of a Novel Angiogenesis Signalling circSCRG1/miR-1268b/NR4A1 Pathway in Atherosclerosis and the Regulatory Effects of TMP-PF In Vitro.Molecules. 2023 Jan 28;28(3):1271. doi: 10.3390/molecules28031271. Molecules. 2023. PMID: 36770940 Free PMC article.
References
-
- Bautch VL (2010) Cancer: Tumour stem cells switch sides. Nature 468, 770–771 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

