Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo
- PMID: 30391357
- PMCID: PMC6348084
- DOI: 10.1016/j.canlet.2018.10.020
Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo
Abstract
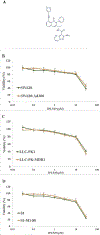
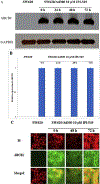
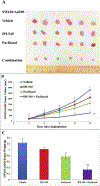
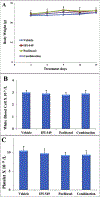
Phosphoinositide 3-kinase gamma isoform (PI3Kγ) plays a critical role in myeloid-derived cells of the immunosuppressive tumor microenvironment. IPI-549, a recently discovered small molecule selective PI3Kγ inhibitor, is currently under immuno-oncology clinical trials in combination with nivolumab, an anti-PD-1 monoclonal antibody immune checkpoint blocker. The purpose of this study is to investigate whether IPI-549 could reverse P-glycoprotein (P-gp)-mediated MDR when combined with chemotherapeutic substrates of P-gp. Cytotoxicity assays showed that IPI-549 reverses P-gp-mediated MDR in SW620/Ad300 and LLC-PK-MDR1 cells. IPI-549 increases the amount of intracellular paclitaxel and inhibits the efflux of paclitaxel out of SW620/Ad300 cells. ABCB1-ATPase assay showed that IPI-549 stimulates the activity of ABCB1-ATPase. IPI-549 does not alter the expression and does not affect the subcellular localization of P-gp in SW620/Ad300 cells. The combination of IPI-549 with paclitaxel showed that IPI-549 potentiates the anti-tumor effects of paclitaxel in P-gp-overexpressing MDR SW620/Ad300 xenograft tumors. With clinical trials beginning to add newly approved immune checkpoint-based immunotherapy into standard-of-care immunogenic chemotherapy to improve patient outcomes, our findings support the rationale of adding IPI-549 to both the chemotherapeutic and immunotherapeutic aspects of cancer combination treatment strategies.
Keywords: ABCB1 transporter; Combination chemotherapy; IPI-549; Immune checkpoint; Multidrug resistance; P-glycoprotein.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
7. Conflict of interests
The authors declare that they have no conflicting interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

