Peroxynitrite nitrates adenine nucleotide translocase and voltage-dependent anion channel 1 and alters their interactions and association with hexokinase II in mitochondria
- PMID: 30391711
- PMCID: PMC6487210
- DOI: 10.1016/j.mito.2018.10.002
Peroxynitrite nitrates adenine nucleotide translocase and voltage-dependent anion channel 1 and alters their interactions and association with hexokinase II in mitochondria
Abstract
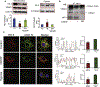
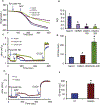
Cardiac ischemia and reperfusion (IR) injury induces excessive emission of deleterious reactive O2 and N2 species (ROS/RNS), including the non-radical oxidant peroxynitrite (ONOO-) that can cause mitochondria dysfunction and cell death. In this study, we explored whether IR injury in isolated hearts induces tyrosine nitration of adenine nucleotide translocase (ANT) and alters its interaction with the voltage-dependent anion channel 1 (VDAC1). We found that IR injury induced tyrosine nitration of ANT and that exposure of isolated cardiac mitochondria to ONOO- induced ANT tyrosine, Y81, nitration. The exposure of isolated cardiac mitochondria to ONOO- also led ANT to form high molecular weight proteins and dissociation of ANT from VDAC1. We found that IR injury in isolated hearts, hypoxic injury in H9c2 cells, and ONOO- treatment of H9c2 cells and isolated mitochondria, each decreased mitochondrial bound-hexokinase II (HK II), which suggests that ONOO- caused HK II to dissociate from mitochondria. Moreover, we found that mitochondria exposed to ONOO- induced VDAC1 oligomerization which may decrease its binding with HK II. We have reported that ONOO- produced during cardiac IR injury induced tyrosine nitration of VDAC1, which resulted in conformational changes of the protein and increased channel conductance associated with compromised cardiac function on reperfusion. Thus, our results imply that ONOO- produced during IR injury and hypoxic stress impeded HK II association with VDAC1. ONOO- exposure nitrated mitochondrial proteins and also led to cytochrome c (cyt c) release from mitochondria. In addition, in isolated mitochondria exposed to ONOO- or obtained after IR, there was significant compromise in mitochondrial respiration and delayed repolarization of membrane potential during oxidative (ADP) phosphorylation. Taken together, ONOO- produced during cardiac IR injury can nitrate tyrosine residues of two key mitochondrial membrane proteins involved in bioenergetics and energy transfer to contribute to mitochondrial and cellular dysfunction.
Keywords: Adenine nucleotide translocase; Cardiac ischemia reperfusion injury; Hexokinase II; Mitochondria; Peroxynitrite; Tyrosine nitration; Voltage-dependent anion channel 1.
Copyright © 2018 Elsevier B.V. and Mitochondria Research Society. All rights reserved.
Conflict of interest statement
disclosures
The authors have nothing to disclose concerning any conflict of interest.
Figures



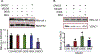
Similar articles
-
Tyrosine nitration of voltage-dependent anion channels in cardiac ischemia-reperfusion: reduction by peroxynitrite scavenging.Biochim Biophys Acta. 2012 Nov;1817(11):2049-59. doi: 10.1016/j.bbabio.2012.06.004. Epub 2012 Jun 15. Biochim Biophys Acta. 2012. PMID: 22709907 Free PMC article.
-
Knockout of VDAC1 in H9c2 Cells Promotes Oxidative Stress-Induced Cell Apoptosis through Decreased Mitochondrial Hexokinase II Binding and Enhanced Glycolytic Stress.Cell Physiol Biochem. 2020 Sep 9;54(5):853-874. doi: 10.33594/000000274. Cell Physiol Biochem. 2020. PMID: 32901466 Free PMC article.
-
Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria.Circ Res. 2003 May 2;92(8):873-80. doi: 10.1161/01.RES.0000069215.36389.8D. Epub 2003 Mar 27. Circ Res. 2003. PMID: 12663490 Free PMC article.
-
The mitochondrial voltage-dependent anion channel 1 in tumor cells.Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2547-75. doi: 10.1016/j.bbamem.2014.10.040. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448878 Review.
-
The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis.Acta Biochim Pol. 2003;50(2):389-404. Acta Biochim Pol. 2003. PMID: 12833165 Review.
Cited by
-
Pyruvate Dehydrogenase and Tricarboxylic Acid Cycle Enzymes Are Sensitive Targets of Traumatic Brain Injury Induced Metabolic Derangement.Int J Mol Sci. 2019 Nov 16;20(22):5774. doi: 10.3390/ijms20225774. Int J Mol Sci. 2019. PMID: 31744143 Free PMC article.
-
N-Terminomic Identification of Intracellular MMP-2 Substrates in Cardiac Tissue.J Proteome Res. 2024 Oct 4;23(10):4188-4202. doi: 10.1021/acs.jproteome.3c00755. Epub 2024 Apr 22. J Proteome Res. 2024. PMID: 38647137 Free PMC article.
-
Nitric oxide-based multi-synergistic nanomedicine: an emerging therapeutic for anticancer.J Nanobiotechnology. 2024 Nov 4;22(1):674. doi: 10.1186/s12951-024-02929-z. J Nanobiotechnology. 2024. PMID: 39497134 Free PMC article. Review.
-
Modulation of peroxynitrite produced via mitochondrial nitric oxide synthesis during Ca2+ and succinate-induced oxidative stress in cardiac isolated mitochondria.Biochim Biophys Acta Bioenerg. 2020 Dec 1;1861(12):148290. doi: 10.1016/j.bbabio.2020.148290. Epub 2020 Aug 20. Biochim Biophys Acta Bioenerg. 2020. PMID: 32828729 Free PMC article.
-
The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches.Basic Res Cardiol. 2023 Nov 8;118(1):48. doi: 10.1007/s00395-023-01018-w. Basic Res Cardiol. 2023. PMID: 37938421 Free PMC article. Review.
References
-
- Allouche M, Pertuiset C, Robert JL, Martel C, Veneziano R, Henry C, dein OS, Saint N, Brenner C, Chopineau J, 2012. ANT-VDAC1 interaction is direct and depends on ANT isoform conformation in vitro. Biochemical and biophysical research communications 429, 12–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

