A maladaptive ER stress response triggers dysfunction in highly active muscles of mice with SELENON loss
- PMID: 30391828
- PMCID: PMC6223234
- DOI: 10.1016/j.redox.2018.10.017
A maladaptive ER stress response triggers dysfunction in highly active muscles of mice with SELENON loss
Abstract
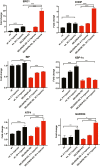
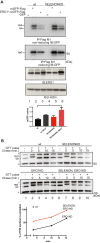
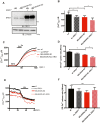
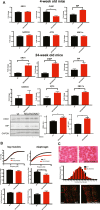
Selenoprotein N (SELENON) is an endoplasmic reticulum (ER) protein whose loss of function leads to human SELENON-related myopathies. SelenoN knockout (KO) mouse limb muscles, however, are protected from the disease, and display no major alterations in muscle histology or contractile properties. Interestingly, we find that the highly active diaphragm muscle shows impaired force production, in line with the human phenotype. In addition, after repeated stimulation with a protocol which induces muscle fatigue, also hind limb muscles show altered relaxation times. Mechanistically, muscle SELENON loss alters activity-dependent calcium handling selectively impinging on the Ca2+ uptake of the sarcoplasmic reticulum and elicits an ER stress response, including the expression of the maladaptive CHOP-induced ERO1. In SELENON-devoid models, ERO1 shifts ER redox to a more oxidised poise, and further affects Ca2+ uptake. Importantly, CHOP ablation in SelenoN KO mice completely prevents diaphragm dysfunction, the prolonged limb muscle relaxation after fatigue, and restores Ca2+ uptake by attenuating the induction of ERO1. These findings suggest that SELENON is part of an ER stress-dependent antioxidant response and that the CHOP/ERO1 branch of the ER stress response is a novel pathogenic mechanism underlying SELENON-related myopathies.
Keywords: Diaphragm dysfunction; ER stress response; SELENON.
Copyright © 2018 The Author. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N.H., Arias C., Lennon C.J., Kluger Y., Dynlacht B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. - PubMed
-
- Arbogast S., Ferreiro A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid. Redox Signal. 2010;12:893–904. - PubMed
-
- Blaauw B., Canato M., Agatea L., Toniolo L., Mammucari C., Masiero E., Abraham R., Sandri M., Schiaffino S., Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:3896–3905. - PubMed
-
- Bonora M., Giorgi C., Bononi A., Marchi S., Patergnani S., Rimessi A., Rizzuto R., Pinton P. Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat. Protoc. 2013;8:2105–2118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

