Mechanisms of formin-mediated actin assembly and dynamics
- PMID: 30392063
- PMCID: PMC6297096
- DOI: 10.1007/s12551-018-0468-6
Mechanisms of formin-mediated actin assembly and dynamics
Abstract
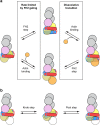
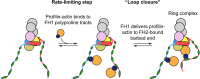
Cellular viability requires tight regulation of actin cytoskeletal dynamics. Distinct families of nucleation-promoting factors enable the rapid assembly of filament nuclei that elongate and are incorporated into diverse and specialized actin-based structures. In addition to promoting filament nucleation, the formin family of proteins directs the elongation of unbranched actin filaments. Processive association of formins with growing filament ends is achieved through continuous barbed end binding of the highly conserved, dimeric formin homology (FH) 2 domain. In cooperation with the FH1 domain and C-terminal tail region, FH2 dimers mediate actin subunit addition at speeds that can dramatically exceed the rate of spontaneous assembly. Here, I review recent biophysical, structural, and computational studies that have provided insight into the mechanisms of formin-mediated actin assembly and dynamics.
Keywords: Actin; Formin; Polymerization; Profilin.
Conflict of interest statement
Conflict of interest
Naomi Courtemanche declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

