Hydroxybiphenylamide GroEL/ES Inhibitors Are Potent Antibacterials against Planktonic and Biofilm Forms of Staphylococcus aureus
- PMID: 30392371
- PMCID: PMC6467803
- DOI: 10.1021/acs.jmedchem.8b01293
Hydroxybiphenylamide GroEL/ES Inhibitors Are Potent Antibacterials against Planktonic and Biofilm Forms of Staphylococcus aureus
Abstract
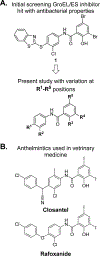
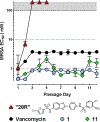
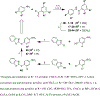
We recently reported the identification of a GroEL/ES inhibitor (1, N-(4-(benzo[ d]thiazol-2-ylthio)-3-chlorophenyl)-3,5-dibromo-2-hydroxybenzamide) that exhibited in vitro antibacterial effects against Staphylococcus aureus comparable to vancomycin, an antibiotic of last resort. To follow up, we have synthesized 43 compound 1 analogs to determine the most effective functional groups of the scaffold for inhibiting GroEL/ES and killing bacteria. Our results identified that the benzothiazole and hydroxyl groups are important for inhibiting GroEL/ES-mediated folding functions, with the hydroxyl essential for antibacterial effects. Several analogs exhibited >50-fold selectivity indices between antibacterial efficacy and cytotoxicity to human liver and kidney cells in cell culture. We found that MRSA was not able to easily generate acute resistance to lead inhibitors in a gain-of-resistance assay and that lead inhibitors were able to permeate through established S. aureus biofilms and maintain their bactericidal effects.
Figures




References
-
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 Centers for Disease Control and Prevention: Atlanta, Georgia, USA, 2013; p 114.
-
- Boucher HW; Talbot GH; Bradley JS; Edwards JE; Gilbert D; Rice LB; Scheld M; Spellberg B; Bartlett J Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis 2009, 48, 1–12. - PubMed
-
- Lewis K Platforms for antibiotic discovery. Nat. Rev. Drug Discov 2013, 12, 371–387. - PubMed
-
- Wright GD; Sutherland AD New strategies for combating multidrug-resistant bacteria. Trends Mol. Med 2007, 13, 260–267. - PubMed
-
- Bjarnsholt T The role of bacterial biofilms in chronic infections. APMIS Suppl 2013, 1–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials

