Oral Ondansetron Administration to Nondehydrated Children With Diarrhea and Associated Vomiting in Emergency Departments in Pakistan: A Randomized Controlled Trial
- PMID: 30392735
- PMCID: PMC6390170
- DOI: 10.1016/j.annemergmed.2018.09.011
Oral Ondansetron Administration to Nondehydrated Children With Diarrhea and Associated Vomiting in Emergency Departments in Pakistan: A Randomized Controlled Trial
Abstract
Study objective: We determine whether single-dose oral ondansetron administration to children with vomiting as a result of acute gastroenteritis without dehydration reduces administration of intravenous fluid rehydration.
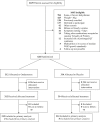
Methods: In this 2-hospital, double-blind, placebo-controlled, emergency department-based, randomized trial conducted in Karachi Pakistan, we recruited children aged 0.5 to 5.0 years, without dehydration, who had diarrhea and greater than or equal to 1 episode of vomiting within 4 hours of arrival. Patients were randomly assigned (1:1), through an Internet-based randomization service using a stratified variable-block randomization scheme, to single-dose oral ondansetron or placebo. The primary endpoint was intravenous rehydration (administration of ≥20 mL/kg of an isotonic fluid during 4 hours) within 72 hours of randomization.
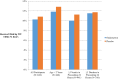
Results: Participant median age was 15 months (interquartile range 10 to 26) and 59.4% (372/626) were male patients. Intravenous rehydration use was 12.1% (38/314) and 11.9% (37/312) in the placebo and ondansetron groups, respectively (odds ratio 0.98; 95% confidence interval [CI] 0.60 to 1.61; difference 0.2%; 95% CI of the difference -4.9% to 5.4%). Bolus fluid administration occurred within 72 hours of randomization in 10.8% (34/314) and 10.3% (27/312) of children administered placebo and ondansetron, respectively (odds ratio 0.95; 95% CI 0.56 to 1.59). A multivariable regression model fitted with treatment group and adjusted for antiemetic administration, antibiotics, zinc prerandomization, and vomiting frequency prerandomization yielded similar results (odds ratio 0.91; 95% CI 0.55 to 1.53). There was no interaction between treatment group and age, greater than or equal to 3 stools in the preceding 24 hours, or greater than or equal to 3 vomiting episodes in the preceding 24 hours.
Conclusion: Oral administration of a single dose of ondansetron did not result in a reduction in intravenous rehydration use. In children without dehydration, ondansetron does not improve clinical outcomes.
Trial registration: ClinicalTrials.gov NCT01870635.
Copyright © 2018 American College of Emergency Physicians. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Oral Ondansetron to Reduce Intravenous Fluid Rehydration: Context Matters.Ann Emerg Med. 2019 Mar;73(3):266-268. doi: 10.1016/j.annemergmed.2018.10.006. Epub 2018 Nov 15. Ann Emerg Med. 2019. PMID: 30447944 No abstract available.
Similar articles
-
Oral Ondansetron Administration to Dehydrated Children in Pakistan: A Randomized Clinical Trial.Pediatrics. 2019 Dec;144(6):e20192161. doi: 10.1542/peds.2019-2161. Epub 2019 Nov 6. Pediatrics. 2019. PMID: 31694979 Clinical Trial.
-
A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis.Ann Emerg Med. 2002 Apr;39(4):397-403. doi: 10.1067/mem.2002.122706. Ann Emerg Med. 2002. PMID: 11919526 Clinical Trial.
-
Oral Ondansetron in Management of Dehydrating Diarrhea with Vomiting in Children Aged 3 Months to 5 Years: A Randomized Controlled Trial.J Pediatr. 2016 Feb;169:105-9.e3. doi: 10.1016/j.jpeds.2015.10.006. Epub 2015 Dec 1. J Pediatr. 2016. PMID: 26654135 Clinical Trial.
-
Effect of ondansetron on vomiting associated with acute gastroenteritis in a developing country: a meta-analysis.Eur J Pediatr. 2020 Aug;179(8):1181-1189. doi: 10.1007/s00431-020-03680-x. Epub 2020 Jun 3. Eur J Pediatr. 2020. PMID: 32495146
-
Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries.Evid Based Child Health. 2013 Jul;8(4):1123-37. doi: 10.1002/ebch.1932. Evid Based Child Health. 2013. PMID: 23877938 Review.
Cited by
-
A Half Century of Oral Rehydration Therapy in Childhood Gastroenteritis: Toward Increasing Uptake and Improving Coverage.Dig Dis Sci. 2020 Feb;65(2):355-360. doi: 10.1007/s10620-019-05921-y. Dig Dis Sci. 2020. PMID: 31797188
-
Oral Ondansetron Administration in Children Seeking Emergency Department Care for Acute Gastroenteritis: A Patient-Level Propensity-Matched Analysis.Ann Emerg Med. 2022 Jan;79(1):66-74. doi: 10.1016/j.annemergmed.2021.06.003. Epub 2021 Aug 11. Ann Emerg Med. 2022. PMID: 34389195 Free PMC article.
-
Oral Ondansetron versus Domperidone for Acute Gastroenteritis Associated Vomiting in Young Children.Cureus. 2019 Sep 12;11(9):e5639. doi: 10.7759/cureus.5639. Cureus. 2019. PMID: 31700742 Free PMC article.
References
-
- Santosham M., Chandran A., Fitzwater S. Progress and barriers for the control of diarrhoeal disease. Lancet. 2010;376:63–67. - PubMed
-
- Kamala C.S., Vishwanathakumar H.M., Shetti P.M. Management of diarrhea in a DTU. Indian Pediatr. 1996;33:856–860. - PubMed
-
- Ibrahim S., Isani Z. Sagodana based verses rice based oral rehydration solution in the management of acute diarrhoea in Pakistani children. J Pak Med Assoc. 1997;47:16–19. - PubMed